- Search
| Ann Coloproctol > Volume 39(2); 2023 > Article |
|
Abstract
Purpose
Methods
Results
Conclusion
Notes
FUNDING
This research was funded by the Fundamental Research Grant Scheme awarded by the Ministry of Higher Education of Malaysia (USIM/FRGS/FPSK/055002/50317).
AUTHOR CONTRIBUTIONS
Conceptualization: RFJ, NHO, NAH; Data curation: RFJ, NHO; Formal analysis: RFJ, NHO; Funding acquisition: RFJ, HAR, NAH; Methodology: NHO; Supervision: RFJ, HAR, NAH; Validation: RFJ, HAR, NAH; WritingŌĆōoriginal draft: NHO; WritingŌĆōreview & editing: RFJ, HAR, NAH. All authors read and approved the final manuscript.
Fig.┬Ā1.
Fig.┬Ā2.

Fig.┬Ā3.
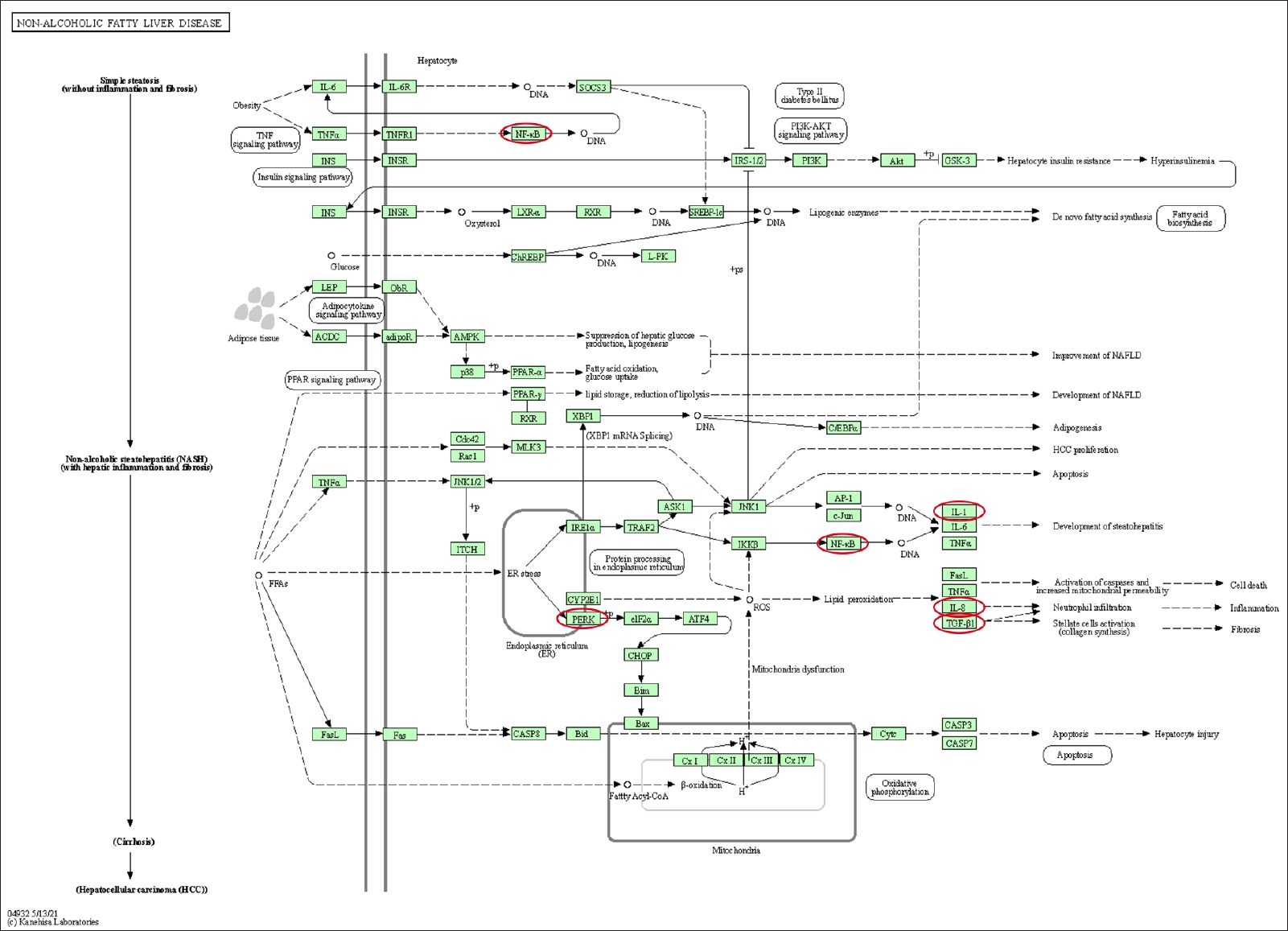
Fig.┬Ā4.
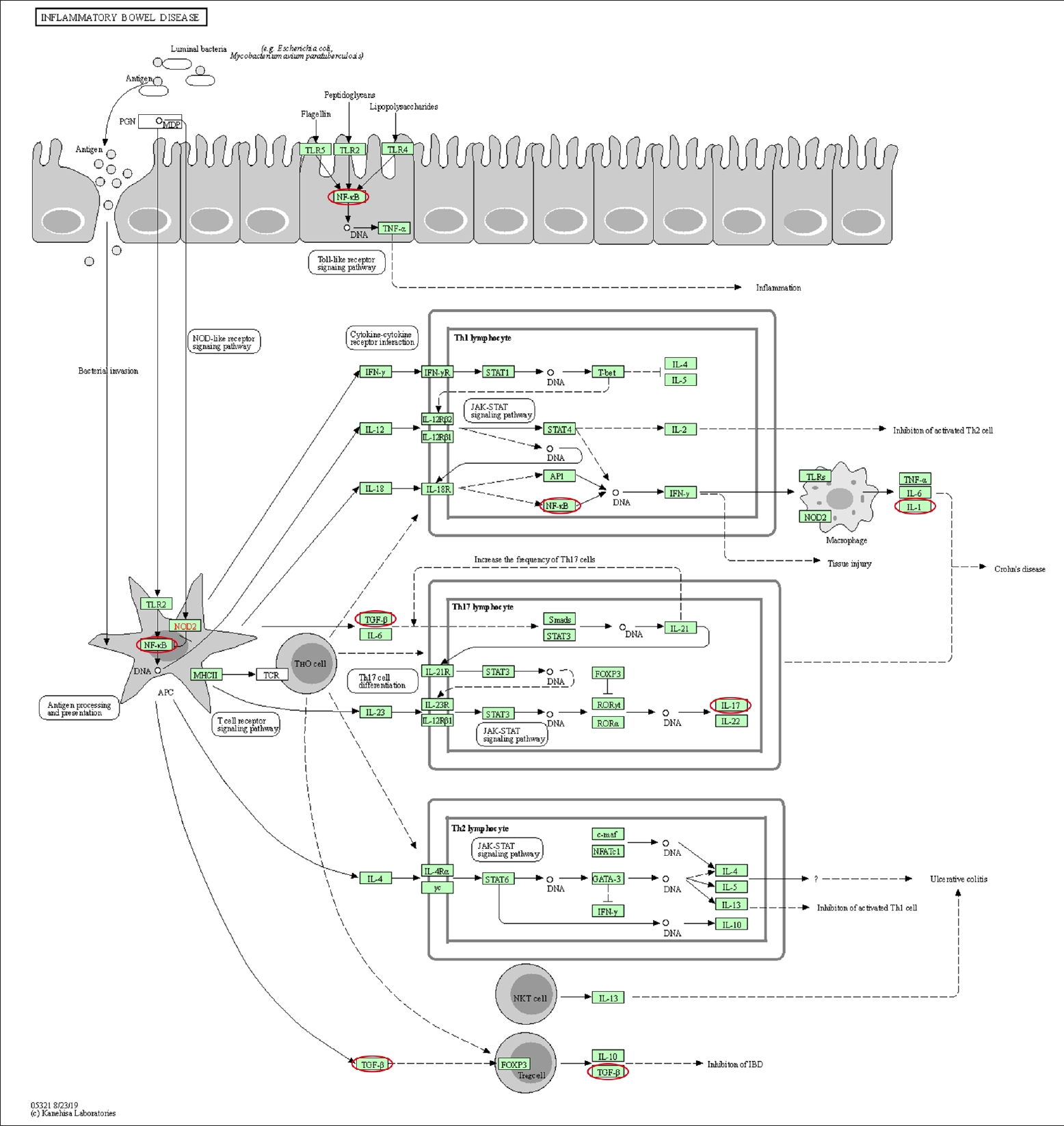
Fig.┬Ā5.
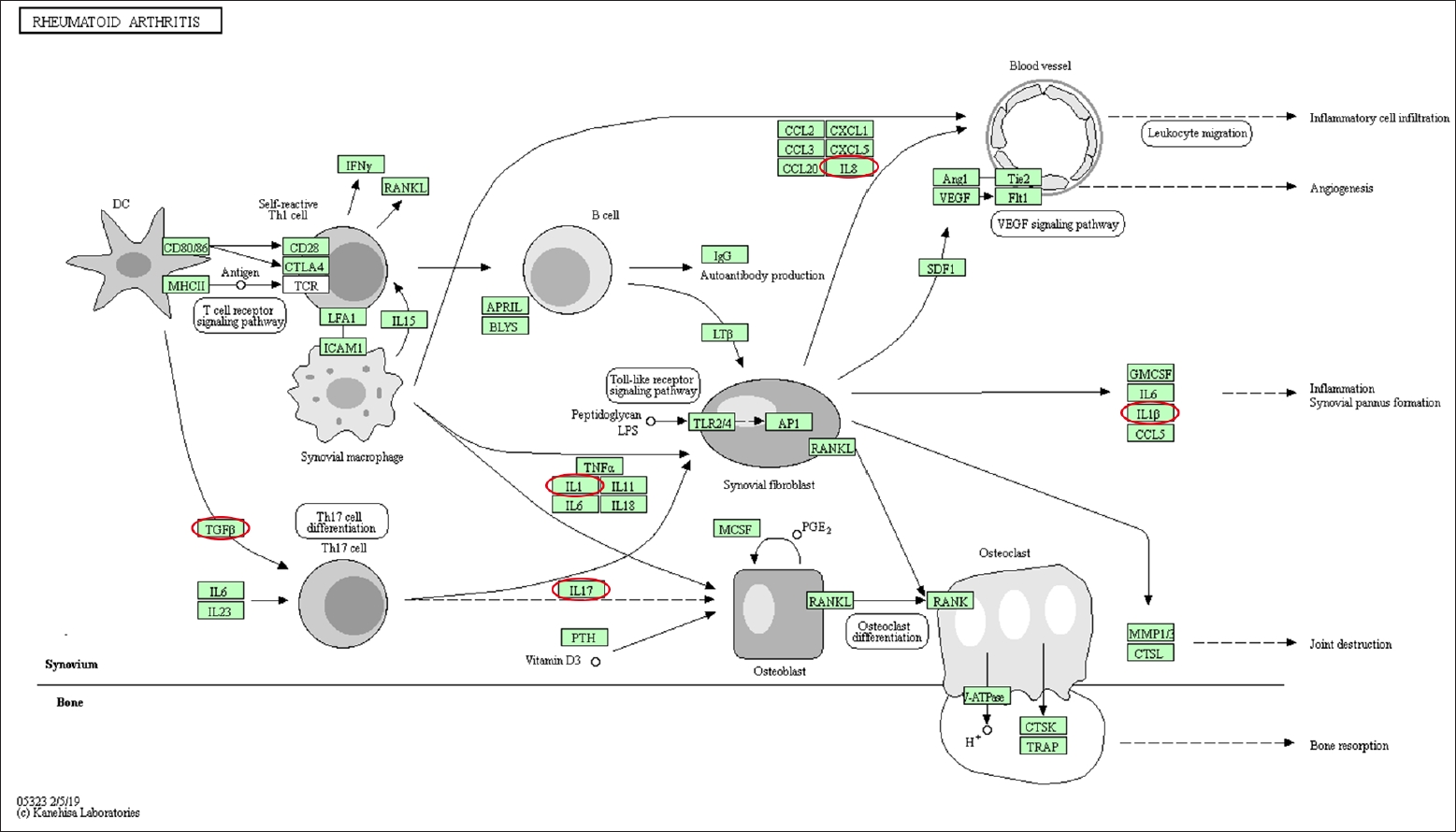
Table┬Ā1.
| Study | Year |
Type of gene |
|
|---|---|---|---|
| Inflammation-related | EMT-related | ||
| Zhang et al. [21] | 2018 | IL-17A | Vimentin, Snail, ╬▒-SMA, E-cadherin |
| Chen et al. [22] | 2013 | NA | Vimentin, TGF-╬▓1, miR-200b, E-cadherin |
| Ratto et al. [23] | 2016 | IL-1╬▓, IL-8 | TGF-╬▓1, Zeb 1, Snail (Snail2), Vimentin, pERK, NF-╬║B, E-cadherin |
EMT, epithelial-mesenchymal transition; IL, interleukin; ╬▒-SMA, smooth muscle alpha-actin; E-cadherin, epithelial cadherin; NA, not applicable; TGF-╬▓1, transforming growth factor beta 1; miR-200b, microRNA-200b; pERK, protein kinase RNA-like endoplasmic reticulum kinase; NF-╬║B, nuclear factor kappa B.
Table┬Ā2.
| Study | Year |
Type and size of sample |
Result | |
|---|---|---|---|---|
| Patient group | Control group | |||
| Zhang et al. [21] | 2018 | Colonic mucosal biopsy tissue of 14 CD patients | Normal intestinal mucosal tissues adjacent to intestinal polyps of 8 patients | ŌĆó The gene and protein expression of IL-17A, vimentin, and ╬▒-SMA in colonic mucosal biopsy tissue of CD patients were significantly higher than those in control group. |
| ŌĆó mRNA level and protein expression of E-cadherin in colonic mucosal tissue from CD patients were significantly lower. | ||||
| ŌĆó No significant differences in expression of Snail between CD and control groups in mRNA level but high protein expression at protein level. | ||||
| Chen et al. [22] | 2013 | 22 Biopsy mucosal samples of IBD patients (11 UC and 11 CD) | 5 Patients with colonic polyps, which pathologically confirm benign adenoma | ŌĆó Gene and protein expression of vimentin and TGF-╬▓1 were significantly higher in IBD group. |
| ŌĆó Gene and protein expression of miR-200b and E-cadherin were significantly lower in IBD group. | ||||
| Ratto et al. [23] | 2016 | 12 Patients with transsphincteric cryptoglandular anal fistula | 3 Patients with fecal incontinence who underwent biopsy of the anal mucosa | ŌĆó Gene expression of IL-1╬▓ and IL-8 for inflammation and cytokine expression were higher than in normal anal mucosa. |
| ŌĆó Gene expression of TGF-╬▓1, Zeb1, Snail2, and vimentin were significantly higher than in normal anal mucosa. | ||||
| ŌĆó Protein expression of pERK and NF-╬║B were significantly higher than in normal anal mucosa. | ||||
| ŌĆó Gene and protein expression of E-cadherin was significantly lower in fistula tract. | ||||
Quantitative real-time polymerase chain reaction was used to analyze gene expression and western blot for protein expression.
CD, Crohn disease; IL, interleukin; ╬▒-SMA, smooth muscle alpha-actin; mRNA, messenger RNA; E-cadherin, epithelial cadherin; TGF-╬▓1, transforming growth factor beta 1; IBD, inflammatory bowel disease; UC, ulcerative colitis; miR-200b, microRNA-200b; pERK, protein kinase RNA-like endoplasmic reticulum kinase; NF-╬║B, nuclear factor kappa B.
Table┬Ā3.
REFERENCES
- TOOLS








