- Search
| Ann Coloproctol > Volume 39(6); 2023 > Article |
|
See commentary "Less is more: lessons from the COVID-19 pandemic in transfusion strategies after colorectal surgery" in Volume 39 on page 445.
Abstract
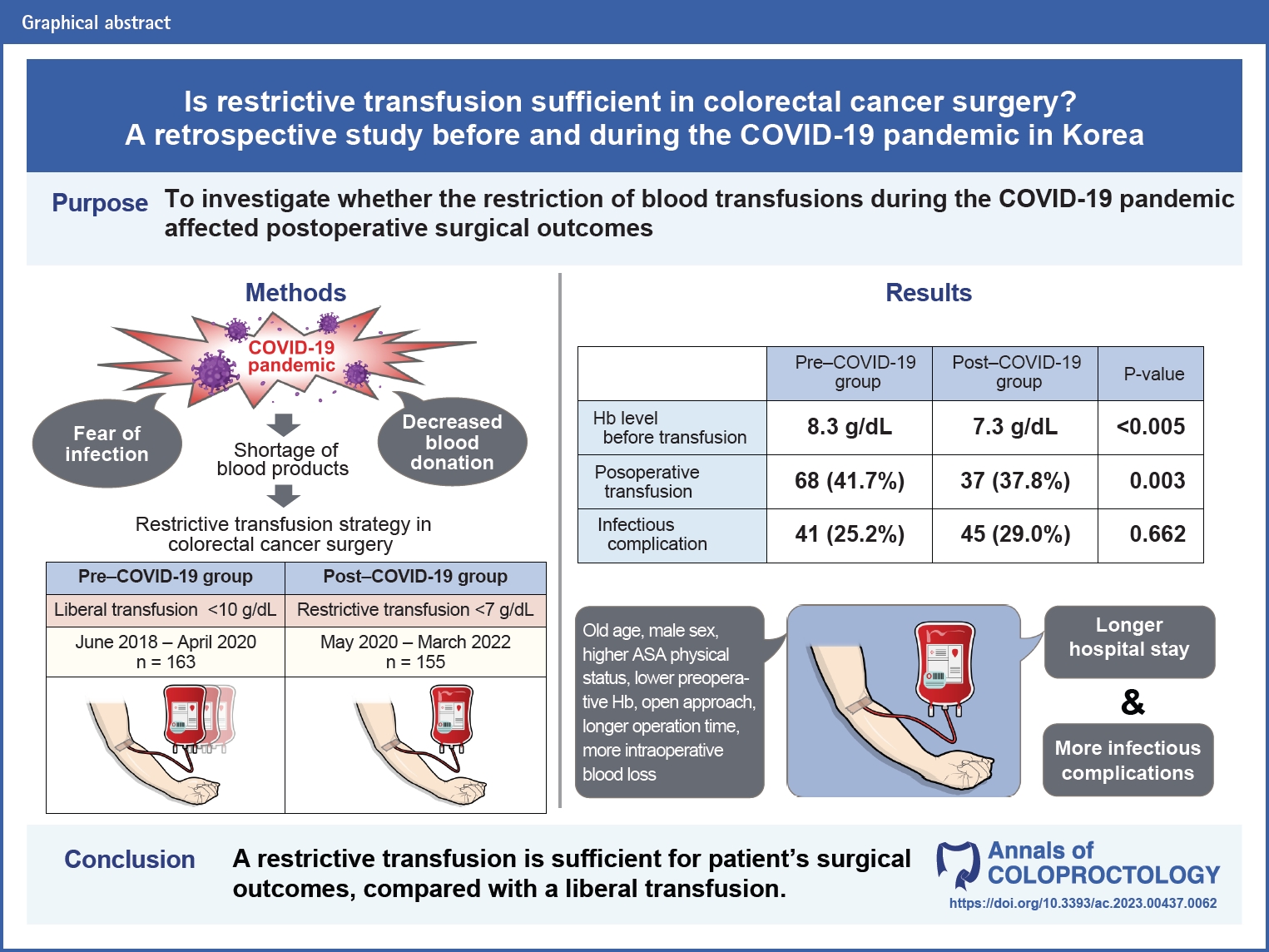
Purpose
Blood transfusion is one of the most common procedures used to treat anemia in colorectal surgery. Despite controversy regarding the adverse effects of blood products, surgeons have maintained standards for administering blood transfusions. However, this trend was restrictive during the COVID-19 pandemic because of a shortage of blood products. In this study, we conducted an analysis to investigate whether the restriction of blood transfusions affected postoperative surgical outcomes.
Methods
Medical records of 318 patients who underwent surgery for colon and rectal cancer at Ewha Womans University Mokdong Hospital between June 2018 and March 2022 were reviewed retrospectively. The surgical outcomes between the liberal and restrictive transfusion strategies in pre– and post–COVID-19 groups were analyzed.
Results
In univariate analysis, postoperative transfusion was associated with infectious complications (odds ratio [OR], 1.705; 95% confidence interval [CI], 1.015–2.865; P=0.044). However, postoperative transfusion was not an independent risk factor for the development of infectious complications in multivariate analysis (OR, 1.305; 95% CI, 0.749–2.274; P=0.348). In subgroup analysis, there was no significant association between infectious complications and the hemoglobin threshold level for the administration of a transfusion (OR, 1.249; 95% CI, 0.928–1.682; P=0.142).
Conclusion
During colorectal surgery, the decision to perform a blood transfusion is an important step in ensuring favorable surgical outcomes. According to the results of this study, restrictive transfusion is sufficient for favorable surgical outcomes compared with liberal transfusion. Therefore, modification of guidelines is suggested to minimize unnecessary transfusion-related side effects and prevent the overuse of blood products.
Anemia can develop in patients undergoing colorectal cancer surgery because of various factors, including cancer-related blood loss, malnutrition, neoadjuvant chemotherapy, systemic inflammation, and intraoperative blood loss. Even though preoperative intravenous iron has been utilized to treat anemia, postoperative transfusion may still be necessary because of the correlation between anemia and postoperative complications, such as anastomotic leakage, which sometimes results in death [1]. However, numerous reports suggest the occurrence of adverse effects from a blood transfusion during oncologic surgery, such as postoperative infectious complications or long-term disease recurrence. In addition, the transfusion volume could affect the surgical outcome due to its immunosuppressive effect. Based on previous studies, efforts have been made to determine the appropriate threshold of hemoglobin (Hgb) levels for postoperative transfusion [1–5].
The liberal transfusion strategy has been adopted as the conventional transfusion practice when the Hgb level is below 10 g/dL to maintain stable blood circulation and enhance blood perfusion at the anastomotic site [1]. However, controversies have emerged concerning the optimal transfusion strategy for anemic patients especially in critical care. These controversies have been described in several randomized controlled studies, which showed the noninferiority of restrictive transfusion [6]. Moreover, this issue extends to anemic patients in the postoperative period following colorectal surgery because of the increasing adverse surgical outcomes resulting from transfusion. Several randomized studies endorse a restrictive transfusion strategy to decrease the disastrous effects of allogeneic blood transfusion [7, 8]. These controversies also contributed to the shift towards patient blood management for elective surgeries, which recommend nontransfusion management such as iron or folate supplements and the stimulation of erythropoiesis in the perioperative period [9]. Accordingly, restrictive transfusion strategy was admitted which involves judicious withholding of blood transfusions in patients without significant bleeding and deferring transfusion until the Hgb level is as low as 7 g/dL [1, 10–12].
During the COVID-19 pandemic, surgeons found it difficult to manage anemia due to decreased blood donations. Fear of infection and restriction of blood compound supply were probable causes for the decrease [13]. As the blood supply became insufficient, many changes were made to the transfusion policy worldwide, including at our center, Ewha Womans University Mokdong Hospital (Seoul, Korea). For transfusions, the recommended Hgb threshold level was lowered to 7.0 g/dL, whereas there had been no specific limitations for transfusion before the spread of COVID-19. The restrictions on transfusions also resulted in insufficiencies in the correction of anemia in the postoperative period for patients who underwent elective and emergency surgery. In this study, we investigated whether changes in blood transfusion policy in the prepandemic and postpandemic eras influenced the occurrence of infectious complications and suggested anemia restoration guidelines for a safe colorectal surgery.
We conducted this study in compliance with the principles of the Declaration of Helsinki. This study was reviewed and approved by the Institutional Review Board of Ewha Womans University Seoul Hospital (No. 2023-06-006-003). The requirement for informed consent was waived due to the retrospective nature of the study.
A total of 318 patients who underwent surgery for stages I to IV colorectal cancer at Ewha Womans University Mokdong Hospital between June 2018 and March 2022 were enrolled.
During the COVID-19 pandemic era, the transfusion policy was restricted due to a blood supply shortage. At our center, the threshold value of transfusion was lowered from the Hgb level of 10 to 7 g/dL. We defined a restrictive transfusion as the deliberate withholding of blood transfusion until the Hgb level reached a threshold as low as 7.0 g/dL [1, 10–12]. The liberal strategy conventionally aims to initiate transfusion at the Hgb level under 10 g/dL. The restrictive transfusion protocol was enforced during the pandemic, whereas the liberal transfusion protocol was used before the spread of COVID-19. In our center, the correction of anemia was usually implemented by allogenic blood transfusion rather than oral or intravenous supplementation of iron in the perioperative period before and after COVID-19 pandemic era.
In our retrospective study, the patients were divided into 2 groups based on the change in transfusion policy prompted by the initiation of the pandemic era. The pre–COVID-19 group comprised patients who underwent surgery before the spread of COVID-19 in Korea from June 2018 to April 2020. The post–COVID-19 group comprised patients who had surgery after the spread of COVID-19 from May 2020 to March 2022.
We retrospectively compared patient characteristics between the pre– and post–COVID-19 groups. The recorded data included demographics (age, sex), American Society of Anesthesiologists (ASA) physical status, diabetes mellitus status, body mass index, carcinogenic embryonic antigen level, preoperative Hgb level, pathological TNM cancer stage, operation type, operation name, operative approach, operation time, intraoperative blood loss, stoma formation, and length of hospital stay. Operations included extended or right hemicolectomy, extended or left hemicolectomy, Hartmann's procedure, anterior resection, low anterior resection or abdominoperineal resection, and subtotal or total colectomy. For patients who received a transfusion, we collected data on the lowest postoperative Hgb level immediately before the administration of blood transfusion.
The primary outcome was short-term infectious complications occurring within 30 days after surgery. Infectious complications included anastomotic leakage, surgical site infection (SSI), intra-abdominal fluid collection, enterocutaneous fistulas, pneumonia, pleural effusion, and urinary tract infection. Anastomotic leakage was defined as disruption of the anastomosis or fluid collection near the anastomotic site found on a follow-up abdominopelvic computed tomography [1, 14]. The follow-up imaging study was performed in patients with clinical evidence of intra-abdominal infection such as high fever, persistent abdominal pain, leukocytosis, or color change in the intra-abdominal drain.
SSIs were defined based on the Centers for Disease Control and Prevention (CDC) criteria. Superficial SSI involves infection of the skin or subcutaneous tissue of the incision site, whereas deep SSI is defined as an infection of the soft tissues below the incision site. Organ/space SSI involves abdominal spaces (e.g., interloop of the small bowel and peritoneal cavity) that have no fistula with the incision [15].
Continuous variables were analyzed using Student t-test and the Mann-Whitney U-test. Pearson chi-square test or Fisher exact test was performed to compare categorical variables. A logistic regression model was used to analyze the independent factors affecting the occurrence of infectious complications and transfusion administration. Statistical significance was set at P<0.05. All data were analyzed using IBM SPSS ver. 20.0 (IBM Corp).
Of the 318 patients, 163 (51.3%) were enrolled in the pre–COVID-19 group and 155 (48.7%) in the post–COVID-19 group. A comparison of the demographic data, intraoperative characteristics, and postoperative variables, including Hgb levels immediately before the administration of transfusion and the occurrence of short-term infectious complications, between the 2 groups is shown in Table 1. The mean postoperative Hgb level before transfusion was 8.3 g/dL in the pre–COVID-19 group, higher than 7.3 g/dL in the post–COVID group (P<0.005). There was a significant reduction in postoperative transfusions during the pandemic period (P=0.003), without significant differences in the remaining variables between the groups. Despite the implementation of restricted transfusions in the post–COVID-19 group, there was no significant difference in the occurrence of infectious complications before and after the spread of COVID-19.
A comparison of baseline characteristics, intraoperative findings, and postoperative outcomes between the transfusion and nontransfusion groups is shown in Table 2. Among the baseline characteristics, advanced age (71.9±14.4 years vs. 66.9±13.2 years, P=0.003), male sex (P=0.003), diabetes mellitus status (P=0.039), higher ASA physical status (P=0.002), lower preoperative Hgb level (10.3±1.6 g/dL vs. 12.2±2.2 g/dL, P<0.001), preoperative transfusion (P<0.001), and intraoperative transfusion (P<0.001) were significantly associated with the implementation of postoperative transfusion. Emergency operation type (P=0.003), open surgical approach (P<0.001), longer operation time (233.2±108.7 minutes vs. 198.2±80.4 minutes, P=0.010), more intraoperative blood loss (342.7±444.3 mL vs. 173.4±171.7 mL, P<0.001), and stoma formation (P=0.013) were observed in the transfusion group within the intraoperative characteristics. The transfusion group also showed a longer hospital stay (16.7±10.9 days vs. 11.8±6.8 days, P<0.001) and more infectious complications (P=0.037) in postoperative outcomes.
Among the patient characteristics, the factors affecting the occurrence of infectious complications were analyzed, as presented in Table 3. In univariate analysis, age (odds ratio [OR], 1.025; 95% confidence interval [CI], 1.006–1.045; P=0.010), ASA physical status (OR, 2.293; 95% CI, 1.323–3.974; P=0.003), preoperative Hgb level (OR, 0.872; 95% CI, 0.779–0.977; P=0.019), and postoperative transfusion (OR, 1.705; 95% CI, 1.015–2.865; P=0.044) were associated with infectious complications. In multivariate analysis, only the ASA physical status (OR, 1.809; 95% CI, 1.000–3.272; P=0.050) revealed a marginal correlation with the occurrence of infectious complications. The change in transfusion policy prompted by the initiation of the COVID-19 was not associated with the infectious complications (OR, 0.967; 95% CI, 0.547–1.710; P=0.908).
In Supplementary Table 1, a subgroup analysis was performed to analyze the correlation between stoma formation and infectious complications among patients who underwent either low anterior resection or Hartmann procedure. This analysis was conducted with the consideration that stoma formation is predominantly indicated for individuals afflicted with lower level colon or rectal cancer. In both univariate and multivariate analysis, stoma formation (OR, 2.500; 95% CI, 1.165–5.365; P=0.019) was significantly associated with infectious complications.
A subgroup analysis was conducted within the transfusion group to investigate whether the administration of liberal or restrictive transfusions was associated with the occurrence of infectious complications (Table 4). In univariate analysis, no risk factors were found to be significantly associated with the occurrence of infectious complications, including the implementation of restrictive or liberal transfusion. In multivariate analysis, the ASA physical status (OR, 3.280; 95% CI, 1.219–8.828; P=0.019) was found to be significantly associated with the occurrence of infectious complications. There was no significant association between infectious complications and the implementation of restrictive blood transfusion, which was withheld until the Hgb level was as low as 7.0 g/dL.
In this study, we evaluated the effect of restrictive versus liberal transfusion on the occurrence of postoperative infectious complications by comparing the pre– and post–COVID-19 groups in terms of the restrictive transfusion policy administered during the spread of COVID-19. Our study demonstrated no significant difference in the occurrence of infectious complications (anastomotic leakage, organ/space SSIs, and overall infectious complications) between the restrictive strategy of the post–COVID-19 group and the liberal strategy of the pre–COVID-19 group. Based on our findings, it seems necessary to reconsider the standard Hgb level for postoperative transfusion.
Controversies surrounding restrictive and liberal transfusion strategies have emerged due to the established adverse effects of blood transfusion on the human immune system, primarily through transfusion-associated immunomodulation [16, 17]. Numerous studies have been published on the negative outcomes of postoperative transfusions and the effects of restrictive or liberal transfusions in patients experiencing postoperative anemia. Recently, the consensus regarding transfusion strategies has changed, with growing evidence suggesting that a restrictive transfusion is not associated with harm compared with a liberal transfusion strategy [10–12, 18, 19]. A systematic meta-analysis of the effects of transfusion on short- and long-term prognosis [20] suggested that postoperative transfusion increases short-term adverse effects, including infectious complications and anastomotic leakage. The negative effects of blood transfusion have led to controversies regarding the necessity of liberal transfusion and the efficacy of restrictive transfusion. Another systematic review and meta-analysis [21] elucidated that restrictive transfusion decreased the risk of serious healthcare-associated infections compared with liberal transfusion. Carson et al. [22] also suggested that liberal transfusion did not reduce mortality or in-hospital morbidity compared with restrictive transfusion, and in another study [5], suggested that there were no differences in long-term mortality between the 2 strategies. Numerous studies have suggested that postoperative transfusion has a negative effect on postoperative outcomes [16, 19, 23], and restrictive transfusion has no significant disadvantages compared with liberal transfusion in the postoperative period [1, 5, 11, 22].
Consistent with previous studies, our study showed that the transfusion group tended to develop more infectious complications than the nontransfusion group (Table 2). The higher incidence of infectious complications in the transfused group can be attributed to the implementation of transfusion and other confounding factors. We hypothesized that confounding factors in the transfusion group would contribute to the results of multivariate analysis. Consequently, the findings from our study could be interpreted as suggesting that transfusion was associated with adverse outcomes, which is consistent with previous studies [19, 24, 25]. While our multivariate analysis, as presented in Table 3, did not identify any risk factors, including the implementation of transfusions, a subsequent subgroup analysis, detailed in Supplementary Table 1, revealed stoma formation as a risk factor for infectious complications. It is speculated that the association between infectious complication and stoma formation would attribute to the higher frequency of emergency operation such as colon perforation or ischemia. It is worth noting that the incidence of anastomotic leakage within our study population remained relatively low. Therefore, the positive correlation between stoma formation and infectious complications, as established in our study, does not offer a comprehensive explanation for the association between stoma formation and anastomotic leakage.
Subgroup analysis within the transfusion group was performed to evaluate the association between restrictive or liberal transfusion and the occurrence of infectious complications. There was no interaction of infectious complication, and the postoperative lowest Hgb level categorized into 2 groups (Hgb <7 g/dL vs. 7 g/dL≤Hgb<10 g/dL), and this finding suggested that the impact on adverse surgical outcomes did not differ between the liberal strategy and restrictive transfusion. Our findings align with previous studies that have consistently insisted on the lack of advantages or even disadvantages of liberal transfusion related to the occurrence of infectious complications compared with the restrictive transfusion strategy [1]. As a result, the administration of transfusions at lower Hgb levels according to the patient’s condition could be considered as an effective practice for managing postoperative anemia, allowing for the optimization of medical resources by minimizing unnecessary transfusions.
The present study has several limitations. First, we enrolled a small population of patients at a single institution. Second, our study findings were limited to a 30-day postoperative period, and potential complications beyond this period were not investigated. Third, there were no data on the oncologic outcomes due to the limited follow-up period. However, the methodological strength of the present study is that we utilized the transition of the blood transfusion policy resulting from the emergence of the COVID-19 pandemic without any change in the treatment strategy. This approach enabled objective evaluation of the association between transfusion and surgical outcomes.
In conclusion, we found that restrictive transfusion was sufficient for patients who underwent colorectal cancer surgery, particularly under circumstances of limited availability of medical resources, such as during the COVID-19 pandemic. The impact of restrictive and liberal transfusions on negative surgical outcomes did not differ. Although the administration of transfusion itself was not independently associated with infectious complications, transfused patients showed more adverse surgical outcomes. It is crucial to make judicious decisions regarding the implementation of transfusion in patients with postoperative anemia by adequately evaluating their overall condition. To prevent the waste of medical resources and unnecessary transfusion, surgeons should consider prudent transfusion practices in the postoperative period by adopting a restrictive transfusion strategy.
SUPPLEMENTARY MATERIALS
Supplementary Table 1.
Subgroup analysis in cases of low anterior resection and Hartmann procedure
Supplementary materials are available from https://doi.org/10.3393/ac.2023.00437.0062.
Table 1.
The comparison of the variables before and after the COVID-19 pandemic era (n=318)
Table 2.
Baseline characteristics and short-term postoperative outcome according to the implementation of transfusion (n=318)
Table 3.
The risk factors of infectious complication in all patients (n=318)
Table 4.
Subgroup analysis in patients with transfusion (n=105)
REFERENCES
1. Ozben V, Stocchi L, Ashburn J, Liu X, Gorgun E. Impact of a restrictive vs liberal transfusion strategy on anastomotic leakage and infectious complications after restorative surgery for rectal cancer. Colorectal Dis 2017;19:772–80.



2. Tang R, Chen HH, Wang YL, Changchien CR, Chen JS, Hsu KC, et al. Risk factors for surgical site infection after elective resection of the colon and rectum: a single-center prospective study of 2,809 consecutive patients. Ann Surg 2001;234:181–9.



3. Lee MR, Hong CW, Yoon SN, Lim SB, Park KJ, Park JG. Risk factors for anastomotic leakage after resection for rectal cancer. Hepatogastroenterology 2006;53:682–6.


4. Akasu T, Takawa M, Yamamoto S, Yamaguchi T, Fujita S, Moriya Y. Risk factors for anastomotic leakage following intersphincteric resection for very low rectal adenocarcinoma. J Gastrointest Surg 2010;14:104–11.



5. Carson JL, Sieber F, Cook DR, Hoover DR, Noveck H, Chaitman BR, et al. Liberal versus restrictive blood transfusion strategy: 3-year survival and cause of death results from the FOCUS randomised controlled trial. Lancet 2015;385:1183–9.


6. Zeroual N, Blin C, Saour M, David H, Aouinti S, Picot MC, et al. Restrictive transfusion strategy after cardiac surgery: role of central venous oxygen saturation trigger: a randomized controlled trial. Anesthesiology 2021;134:370–80.


7. So-Osman C, Nelissen R, Brand R, Faber F, Slaa RT, Stiggelbout A, et al. The impact of a restrictive transfusion trigger on post-operative complication rate and well-being following elective orthopaedic surgery: a post-hoc analysis of a randomised study. Blood Transfus 2013;11:289–95.


8. McIntyre L, Hebert PC, Wells G, Fergusson D, Marshall J, Yetisir E, et al. Is a restrictive transfusion strategy safe for resuscitated and critically ill trauma patients? J Trauma 2004;57:563–8.


9. Murphy MF, Goodnough LT. The scientific basis for patient blood management. Transfus Clin Biol 2015;22:90–6.


10. Salpeter SR, Buckley JS, Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a meta-analysis and systematic review. Am J Med 2014;127:124–31.


11. Carless PA, Henry DA, Carson JL, Hebert PP, McClelland B, Ker K. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev 2010;(10):CD002042.

12. Carson JL, Stanworth SJ, Roubinian N, Fergusson DA, Triulzi D, Doree C, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev 2016;10:CD002042.



13. Al-Riyami AZ, Burnouf T, Wood EM, Devine DV, Oreh A, Apelseth TO, et al. International Society of Blood Transfusion survey of experiences of blood banks and transfusion services during the COVID-19 pandemic. Vox Sang 2022;117:822–30.


14. Bertelsen CA, Andreasen AH, Jørgensen T, Harling H, Danish Colorectal Cancer Group. Anastomotic leakage after anterior resection for rectal cancer: risk factors. Colorectal Dis 2010;12:37–43.

15. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR, The Hospital Infection Control Practices Advisory Committee. Guideline for prevention of surgical site infection, 1999. Am J Infect Control 1999;27:97–132.


16. Kaneko M, Sasaki S, Ishimaru K, Terai E, Nakayama H, Watanabe T. The impact of perioperative allogeneic blood transfusion on survival in elderly patients with colorectal cancer. Anticancer Res 2015;35:3553–8.

17. Khanbhai M, Shah M, Cantanhede G, Ilyas S, Richards T. The problem of anaemia in patients with colorectal cancer. ISRN Hematol 2014;2014:547914.




18. Gorgun E, Ozben V, Stocchi L, Ozuner G, Liu X, Remzi F. Impact of transfusion threshold on infectious complications after ileal pouch-anal anastomosis. J Gastrointest Surg 2016;20:343–50.



19. Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg 2012;256:235–44.


20. Pang QY, An R, Liu HL. Perioperative transfusion and the prognosis of colorectal cancer surgery: a systematic review and meta-analysis. World J Surg Oncol 2019;17:7.




21. Rohde JM, Dimcheff DE, Blumberg N, Saint S, Langa KM, Kuhn L, et al. Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA 2014;311:1317–26.



22. Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011;365:2453–62.



23. Mynster T, Christensen IJ, Moesgaard F, Nielsen HJ, Danish RANX05 Colorectal Cancer Study Group. Effects of the combination of blood transfusion and postoperative infectious complications on prognosis after surgery for colorectal cancer. Br J Surg 2000;87:1553–62.