- Search
| Ann Coloproctol > Epub ahead of print |
|
Abstract
Purpose
Methods
Results
Notes
AUTHOR CONTRIBUTIONS
Conceptualization: DST, US, DS; Data curation: SM, AV; Formal analysis: SM, AV; Investigation: all authors; Methodology: DST, US, DS; Project administration: DST, US, DS; Software: SM, AV; Supervision: DST, US, DS; Validation: DST, US, DS; Visualization: all authors; WritingŌĆōoriginal draft: SM; WritingŌĆōreview & editing: SM, AV. All authors read and approved the final manuscript.
All authors read and approved the final manuscript.
Fig.┬Ā1.
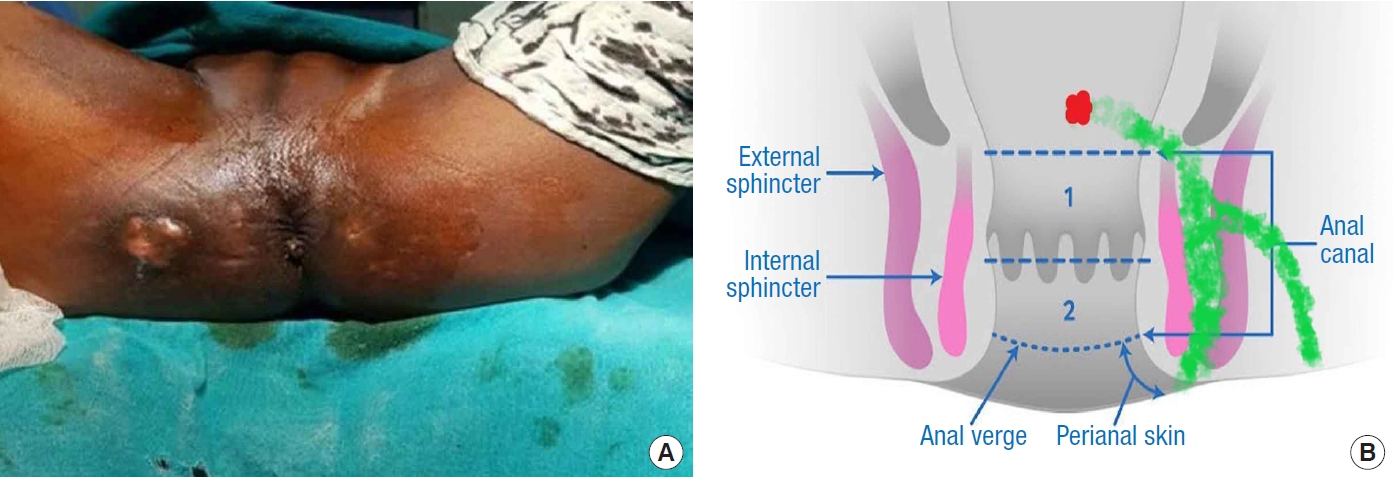
Fig.┬Ā2.

Fig.┬Ā3.

Fig.┬Ā4.
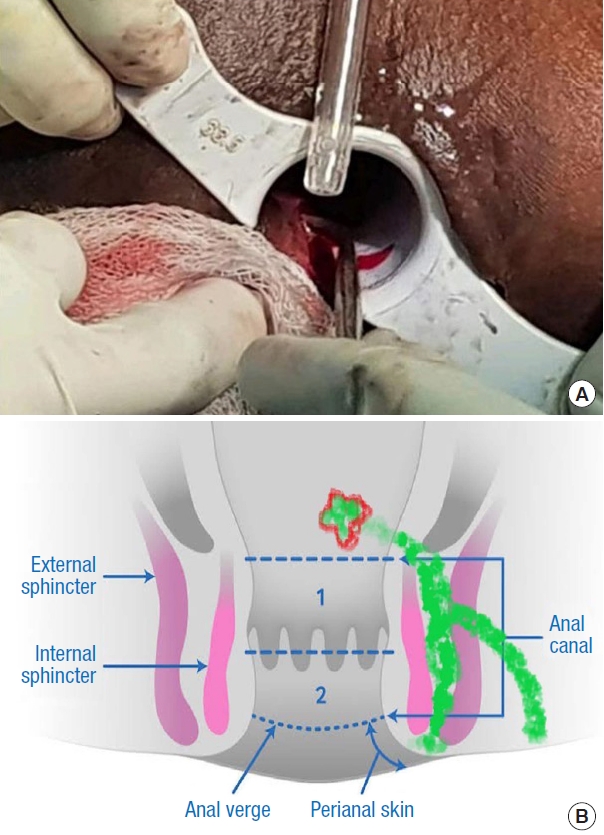
Fig.┬Ā5.
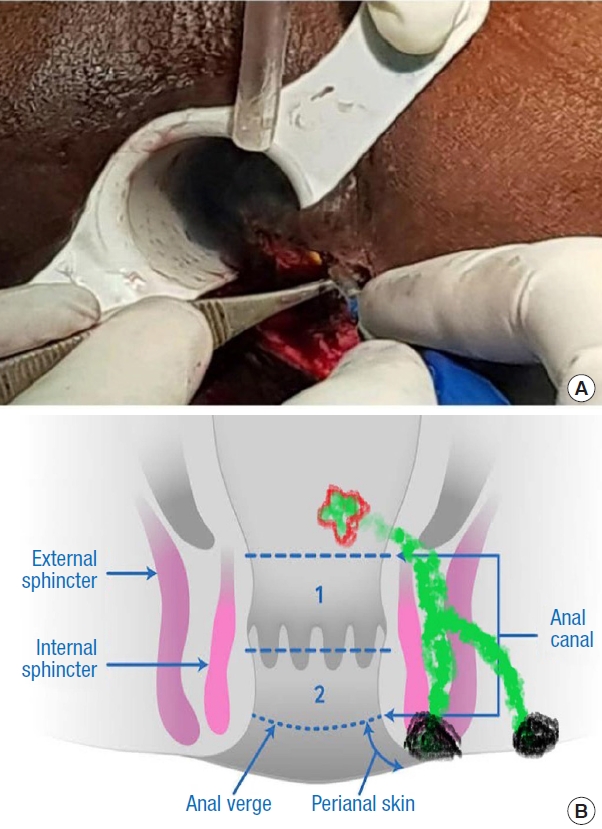
Fig.┬Ā6.
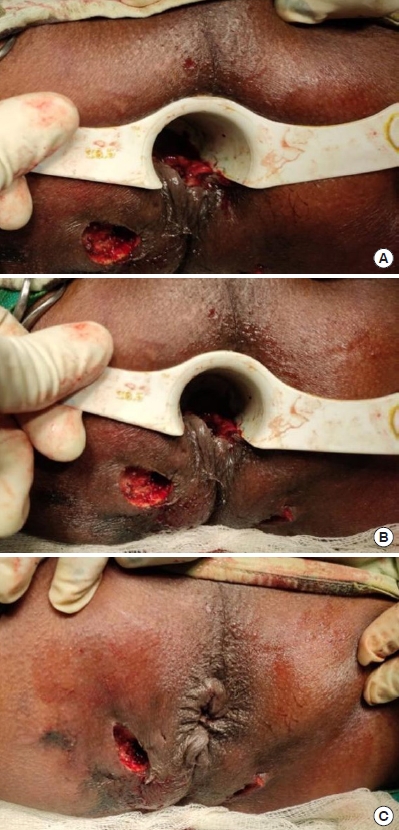
Fig.┬Ā7.
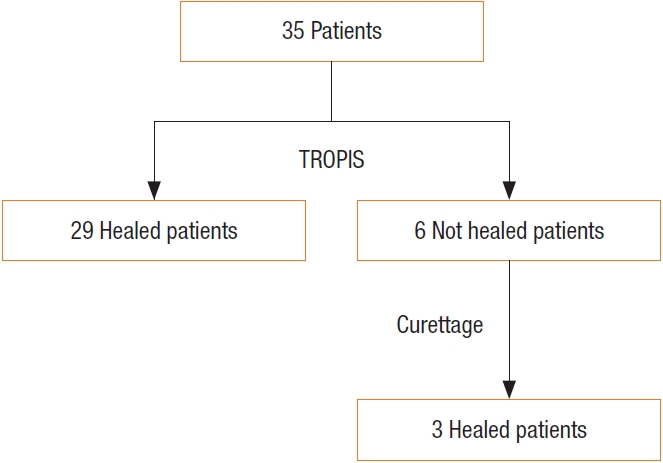
Table┬Ā1.
Table┬Ā2.
| Study | Procedure | No. of patients | Fistula healing rate (%) | Incontinence |
|---|---|---|---|---|
| Champagne et al. [9] (2006) | AFP | 36 | 83.3 | No significant changea |
| Meinero and Mori [10] (2011) | VAAFT | 136 | 87.1 | No significant changea |
| Giamundo et al. [11] (2015) | FiLaC | 45 | 71.1 | No significant changea |
| Rojanasakul et al. [13] (2007) | LIFT | 18 | 94.6 | No significant changea |
| Garg and Garg [14] (2015) | PERFACT | 51 | 90.9 | No significant changea |
| Farag et al. [15] (2019) | 1-Stage fistulectomy with primary sphincter repair | 175 | 90.9 | 2.9% |
| Garg [5] (2017) | TROPIS | 61 | 90.4 | No significant changea |
| Huang et al. [16] (2021) | TROPIS + IOEAUS | 48 | 93.4 | No significant changea |
| Li et al. [17] (2022) | TROPIS | 41 | 85.3 | No significant changea |
| Garg et al. [8] (2021) | TROPIS | 325 | 78.4b | No significant changea |
| This study | TROPIS | 35 | 91.4 | No significant changec |
AFP, anal fistula plug; VAAFT, video-assisted anal fistula treatment; FiLaC, fistula-tract laser closure; LIFT, ligation of intersphincteric fistula tract; PERFACT, proximal superficial cauterization, emptying regularly fistula tracts and curettage of tracts; TROPIS, transanal opening of the intersphincteric space; IOEAUS, intraoperative endoanal ultrasonography.
REFERENCES
- TOOLS
-
METRICS

-
- 2 Crossref
- Scopus
- 2,046 View
- 135 Download
- Related articles in ACP