- Search
| Ann Coloproctol > Volume 40(1); 2024 > Article |
|
Abstract
Purpose
Methods
Results
Notes
Author contributions
Conceptualization: MSC, SHY; Data curation: MSC; Formal analysis: MSC; Investigation: MSC, SCL; Methodology: MSC, SCL; Project administration: WYL; Resources: MSC; Software: MSC; Supervision: JKS, YAP, JH, YBC, HCK; Validation: MSC; Visualization: SCL; Writing–original draft: MSC; Writing–review & editing: all authors. All authors read and approved the final manuscript.
Fig. 1.
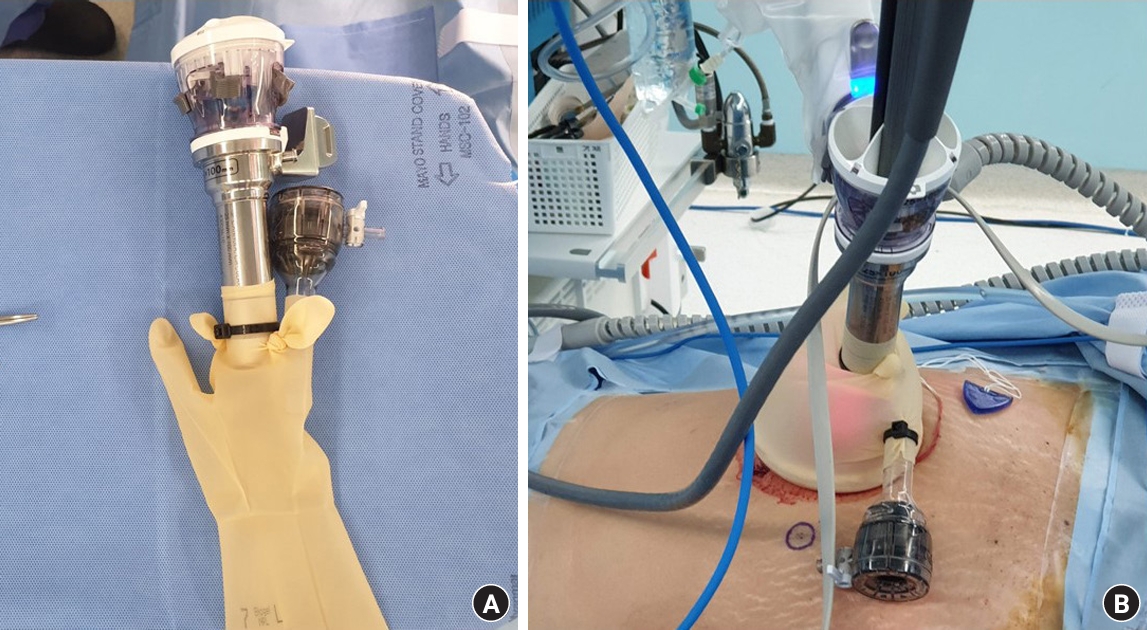
Fig. 2.

Fig. 3.
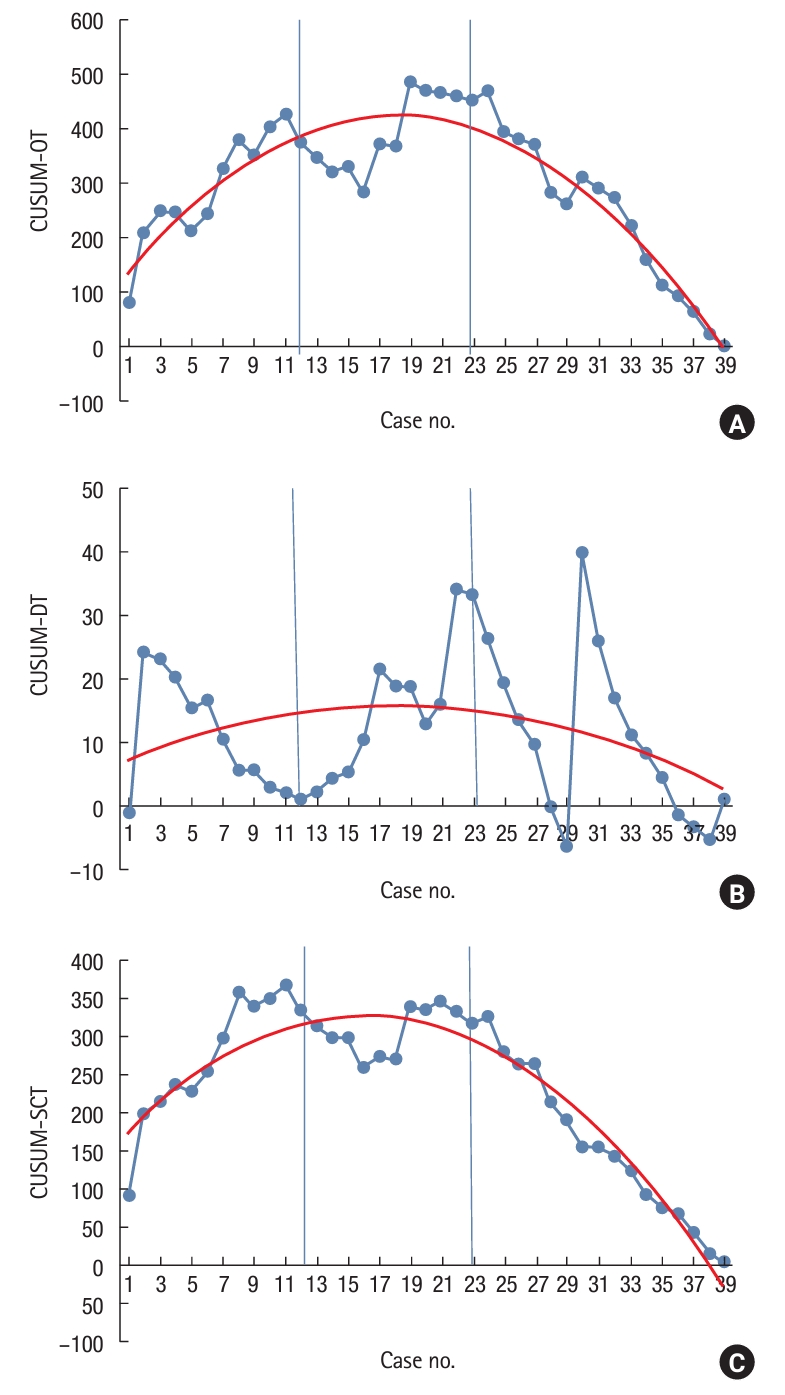
Table 1.
Table 2.
Values are presented as mean±standard deviation.
P-values for post hoc tests: aphase 1 vs. phase 2, P=0.203; phase 1 vs. phase 3, P=0.001; phase 2 vs. phase 3, P=0.260; bphase 1 vs. phase 2, P>0.999; phase 1 vs. phase 3, P>0.999; phase 2 vs. phase 3, P=0.708; cphase 1 vs. phase 2, P=0.011; phase 1 vs. phase 3, P<0.001; phase 2 vs. phase 3, P=0.403.
Table 3.
| Complication | Phase 1 | Phase 2 | Phase 3 |
|---|---|---|---|
| Postoperative ileus | 0 | 0 | 2 |
| Postoperative bleeding | 1 | 0 | 1 |
| JP color change due to chyle | 1 | 0 | 0 |
| Urinary retention | 0 | 0 | 3 |
Table 4.
Values are presented as mean±standard deviation.
RHC, right hemicolectomy; eRHC, extended RHC; LHC, left hemicolectomy; eLHC, extended LHC; AR, anterior resection.
P-value for post hoc tests: aRHC vs. LHC, P=0.777; RHC vs. AR, P=0.001; LHC vs. AR, P=0.002; bRHC vs. LHC, P=0.171; RHC vs. AR, P>0.999; LHC vs. AR, P=0.093; cRHC vs. LHC, P>0.999; RHC vs. AR, P<0.014; LHC vs. AR, P=0.036.
REFERENCES
- TOOLS








