- Search
| Ann Coloproctol > Volume 39(4); 2023 > Article |
|
Abstract
Purpose
Obesity has been known to contribute to technical difficulties in surgery. Until now, body mass index (BMI) has been used to measure obesity. However, there are reports that BMI does not always correspond to the visceral fat. Recently, bioelectrical impedance analysis (BIA) has been used for body composition analysis. This study aimed to evaluate the usefulness of the body composition index obtained using a BIA device in predicting short-term postoperative outcomes.
Methods
Data of patients who underwent elective major colorectal surgery using minimally invasive techniques were reviewed retrospectively. Body composition status was recorded using a commercial BIA device the day before surgery. The relationship between BMI, body composition index, and short-term postoperative outcomes, including operative time, was analyzed.
Results
Sixty-six patients were enrolled in this study. In the correlation analysis, positive correlation was observed between BMI and body composition index. BMI and body composition index were not associated with short-term postoperative outcomes. Percent body fat (odds ratio, 4.226; 95% confidence interval [CI], 1.064–16.780; P=0.041) was found to be a statistically significant factor of prolonged operative time in the multivariate analysis. Correlation analysis showed that body fat mass was related to prolonged operative time (correlation coefficients, 0.245; P=0.048). In the area under curve analysis, body fat mass showed a statistically significant predictive probability for prolonged operative time (body fat mass: area, 0.662; 95% CI, 0.531–0.764; P=0.024).
Obesity is known to contribute to technical difficulties in performing surgery and postoperative morbidities in laparoscopic colorectal surgery [1–3]. Although, in a systematic review involving 23,649 patients, Hotouras et al. [4] concluded that laparoscopic colorectal resection is safe and technically feasible in terms of postoperative morbidities in patients with obesity, longer operative times in patients with obesity who underwent laparoscopic colorectal surgery have been reported [1, 2, 5–19]. Excessive visceral fat can interfere with finding and maintaining an accurate dissection plane, particularly in minimally invasive surgery [17, 20].
Body mass index (BMI) has been used to measure obesity previously [21]. However, there are reports that BMI does not always correspond to the visceral fat, which can be actually related to short-term postoperative outcomes [22, 23]. Therefore, measurement of visceral fat area (VFA) using computed tomography (CT) has been proposed as an alternative to BMI to more accurately predict short-term postoperative outcomes in laparoscopic colorectal surgery [1, 11, 19, 24, 25]. However, measuring VFA using a CT scan is a time-consuming task and requires the use of specific software; moreover, its cost-effectiveness and usefulness in actual clinical practice is still uncertain [26].
More recently, bioelectrical impedance analysis (BIA) has been used for body composition analysis. This approach uses a simple and noninvasive device that can analyze body composition, such as skeletal muscle mass, water content, and body fat mass, by measuring body resistance and capacitance according to changes in the current [27]. In the present study, we aimed to evaluate the usefulness of a body composition index obtained by using a BIA device to predict the short-term postoperative outcomes in patients undergoing minimally invasive colorectal surgery.
This study was reviewed and approved by the Institutional Review Board of Ewha Womans University Seoul Hospital (No. 2022-04-010). The requirement for informed consent from patients was waived due to the retrospective nature of the study.
From June 2021 to February 2022, data of patients who underwent elective major colorectal surgery for colorectal cancer using minimally invasive techniques performed by a single surgeon were retrospectively reviewed. Among the 103 patients, those without a BIA index (n=16) and those without follow-up data indicating short-term postoperative outcomes for at least 1 month (n=3) were excluded. In addition, those who had simultaneous resection (n=16) and subtotal colectomy (n=2) were excluded to determine the accurate correlation between body composition index and operative time. Ultimately, the data from 66 patients were included in the study.
At our institution, patient height and weight are recorded at the time of admission. After bowel preparation on the day before surgery, body composition status is recorded using a commercial BIA device (InBody 270, Inbody Co Ltd) in patients who are scheduled for elective major colorectal surgery. To measure body composition, the patient stands barefoot on a scale and holds the machine’s hand, following simple instructions from the manufacturer. The BIA device used in this study could evaluate body composition and could perform muscle-fat (skeletal muscle mass, body fat mass) and obesity (percent body fat) analyses.
The surgeries of all enrolled patients were performed by an experienced surgeon with more than 300 cases in performing both laparoscopic and robotic surgeries. In most patients, D3 lymphadenectomy was performed [28]. Data on patients’ characteristics were retrospectively reviewed from the medical records. Operative time, intraoperative blood loss, time to diet, time to gas passing, postoperative hospital stay, postoperative morbidity, and readmission rate within postoperative 30 days, which indicates short-term postoperative outcomes, were recorded. Complications were further classified according to the Clavien-Dindo surgical complication grading system [29]. In cases of robotic surgery, docking time was included in the operative time.
Patients with BMI of ≥25 and <25 kg/m2 were classified into the BMI-high and BMI-low groups, respectively [21]. Patients were further divided according to their body composition index using the mean of the data, because, to our knowledge, there are currently no standard values for body fat mass, percentage body fat, and skeletal muscle mass. In addition, because all of the body composition indexes and operative time showed a normal distribution, the mean value of each variable could be used as the cutoff value. The mean values of body fat mass, percent body fat, and skeletal muscle index were 18.5 kg, 29.1%, and 23.6 kg, respectively. Prolonged operative time was defined as >210 minutes for laparoscopic surgery and >240 minutes for robotic surgery, which was based on the mean value obtained from each group in our study as robotic surgery generally resulted in longer operative times.
Continuous variables were compared using the Student t-test and are presented as means with standard deviations or medians with interquartile ranges. Categorical variables were analyzed using the chi-square test or Fisher exact test and are presented as frequencies. A logistic regression model was used to evaluate the factors associated with prolonged operative time. Variables with P-values of <0.10 in univariate analysis were included in a multivariate analysis. Pearson correlation coefficient (r) was used to determine the correlation between operative time and other continuous variables (BMI, body fat mass, percent body fat, and skeletal muscle mass). Receiver operating characteristic (ROC) curve analysis was performed to determine the area under the ROC curve. A P-value of <0.05 was considered statistically significant. All data were analyzed using IBM SPSS ver. 20 (IBM Corp).
In the correlation analysis, positive correlation was observed between BMI and body composition index. Body fat mass was most strongly correlated with BMI (r=0.782, P<0.001). Scatterplots of these associations are shown in Fig. 1.
Of the 66 patients, 44 (66.7%) were enrolled in the BMI-low group and 22 (33.3%) in the BMI-high group. A comparison of the patient characteristics and short-term outcomes between the groups is shown in Table 1. The mean BMI was 21.8 kg/m² in the BMI-low group and 26.9 kg/m² in the BMI-high group. Patients’ baseline demographics, such as age, sex, American Society of Anesthesiologists physical status grade, Eastern Cooperative Oncology Group performance scale score, and smoking and prior abdominal surgery history, were not significantly different. Among the body composition indexes, body fat mass was significantly higher in the BMI-high group (BMI-low, 15.9±4.6 kg; BMI-high, 23.7±6.6 kg; P<0.001). Tumor location, surgical approach, operative method, and pathological status were not different between the 2 groups. Intraoperative blood loss, time to diet, time to gas passing, postoperative hospital stay, postoperative morbidity, and readmission rates did not differ between the 2 groups. For patients in the BMI-high group, the operative time was prolonged but was not statistically different (BMI-low, 213.6±46.7 minutes; BMI-high, 231.8±48.5 minutes; P=0.146). BMI and body composition index were not associated with complications (Table 2).
Among the patient characteristics, factors that could be related to operative time were analyzed. In the univariate analysis, body fat mass, percent body fat, tumor location, T category, and N category were associated with prolonged operative time. In the multivariate analysis, percent body fat, tumor location, and status of lymph node metastasis were associated with prolonged operative time (Table 3).
A subgroup analysis was performed to identify an accurate indicator of prolonged operative time. In all patients, body fat mass was related to prolonged operative time, although the correlation was weak (r=0.245; P=0.048). There was no correlation between body composition index and operative time in each group according to tumor location. However, in patients with lymph node metastasis, BMI and body fat mass were positively associated with operative time (BMI: r=0.441, P=0.031; body fat mass: r=0.404, P=0.050). In addition, in the robotic surgery group, body fat mass tended to be associated with operative time (r=0.415, P=0.055) (Table 4).
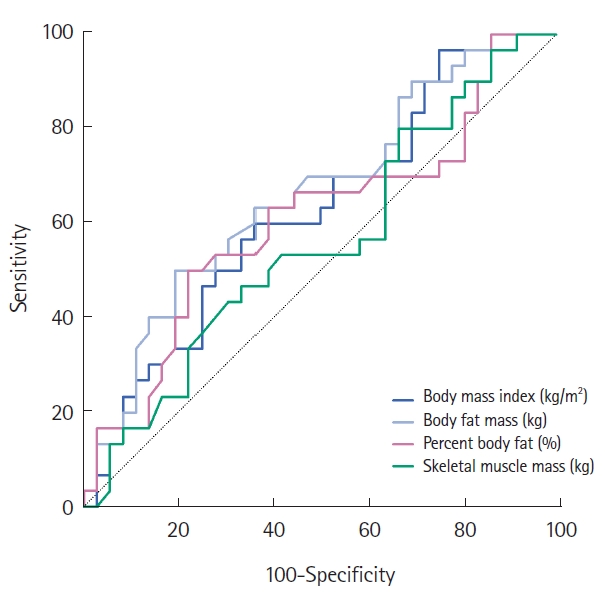
Only body fat mass showed a statistically significant predictive probability for prolonged operative time (body fat mass: area, 0.662; 95% confidence interval, 0.531–0.764; P=0.024). BMI was not a predictive indicator for prolonged operative time (Table 5, Fig. 2). The ROC curves comparing the body fat mass and BMI in the laparoscopic and robotic surgery groups are shown in Fig. 3. Body fat mass was more reliable than BMI in patients who underwent robotic surgery, although it was not statistically significant.
In our study, the BIA device, which measures the body composition index, showed a positive correlation with BMI. However, BMI and body composition index were not associated with short-term postoperative outcomes in patients who underwent minimally invasive colorectal surgery. In terms of operative time, percent body fat, among the body composition indexes, was associated with prolonged operative time in the multivariate analysis. In addition, body fat mass was a significant factor for operative time in the ROC curve analysis. The results of this study may suggest that the body composition index with BIA can be used to replace BMI in predicting prolonged operative time.
However, we thought that our data could not demonstrate the usefulness of the body composition index, compared with BMI, in clearly predicting prolonged operative time. Although some factors were significantly related to operative time, the statistical significance was very low and the correlation coefficients were also weak. This could be due to small sample size; however, there is the limitation that body composition index could calculate the entire body fat mass and percent body fat instead of only trunk fat volume which were related with intra-abdominal surgery. The body composition index for predicting prolonged operative time may be used with caution.
We would like to identify more predictive markers of short-term outcomes, other than BMI. BMI is convenient to use, but in actual surgery, visceral fat is more related to intra-abdominal surgery, as compared to BMI, which does not always correspond to the VFA [22, 23]. In addition, the BMI classification, which is used to define obesity, differs between Western and Asian population [21, 22]. Moreover, Asian population have more pronounced abdominal obesity than Europeans, despite both having similar BMI values [30]. Therefore, the VFA analysis using CT scans, which can measure cross-sectional images, has been studied to predict short-term outcomes [1, 11, 19, 24, 25]. However, measuring the area of visceral fat with a preoperative CT scan is also a time-consuming task despite the availability of free programs [26].
Recently, BIA was used to evaluate body composition indices. It is simple, noninvasive, easy to perform, and allows the analysis of other factors, including body fat mass, percent body fat, and skeletal muscle mass. This rapid and cost-effective device can be used repetitively and without any risk [31]. In various fields, there have been many previous studies that utilize the body composition index with a BIA device [32–38]. However, there have been no studies on other indicators of short-term outcomes and operative time using the BIA device, except for skeletal muscle mass, in patients who had undergone colorectal surgery. Shiomi et al. [39] reported that trunk fat volume measured by using a BIA device can be a useful parameter for the evaluation of obesity and a predictor of complications after gastrectomy.
In our study, BMI and body composition index were not associated with short-term postoperative outcomes. Many studies have reported the relationship between obesity and postoperative outcomes. Some surgeons reported the conversion rate to open surgery, anastomotic leakage rate, and complication rate to be greater in patients with obesity than in those without obesity [2, 3]. However, Horouras et al. [4] reported no significant differences in intraoperative blood loss, overall postoperative morbidity, anastomotic leakage rate, reoperation rate, mortality rate, and the number of retrieved lymph nodes between obese and nonobese groups who underwent colorectal surgery in their meta-analysis, which included 17,895 and 5,754 patients without and with obesity, respectively. Our study results were consistent with those obtained by the previous systematic analysis. With the adoption of minimally invasive surgery in general, better results can be achieved with advanced technology and tools, even in patients with obesity.
However, the body composition index has shown promise as a predictive marker for prolonged operative times. The amount of body fat may be more related to prolonged operative time than BMI. Interestingly, the body fat mass was a more reliable predictor of prolonged operative time in patients who underwent robotic surgery than in those who underwent laparoscopic surgery, although the difference was not statistically significant. In patients enrolled in this study, all robotic surgeries were performed with the da Vinci SP (single port, Intuitive Surgical Inc). Visceral fat may more likely affect the operative time in patients undergoing robotic surgery due to the narrow surgical field and absence of an energy device.
This study had several limitations. First, because the analysis was performed retrospectively at a single center, a relatively small number of patients were included. Therefore, definitive conclusions cannot be drawn from this study. Second, this study lacked long-term follow-up data. A study that includes follow-up data collected using BIA after surgery is necessary to determine the relationship between body composition status and long-term outcomes. However, to the best of our knowledge, our study has an advantage, as we were able to identify the correlation between the body composition index and operative time in patients undergoing minimally invasive colorectal surgery using a noninvasive, reliable, and reproducible device.
In conclusion, the body composition index measured by using a BIA device can be a reliable marker for predicting prolonged operative time, instead of BMI, even though it did not show a difference in short-term outcomes. The body composition index can be used as a predictive marker for prolonged operative time.
Notes
Author contributions
Conceptualization: KHK, HSK, GTN; Data curation: HSK, GTN; Formal analysis: HSK, GTN; Investigation: all authors; Methodology: all authors; Project administration: KHK; Resources: all authors; Software: HSK, GTN; Supervision: KHK; Validation: all authors; Writing–original draft: HSK; Writing–review & editing: all authors. All authors read and approved the final manuscript.
Fig. 1.
A graph showing the correlation between body mass index (BMI) and body composition index. (A) Body fat mass (r=0.782, P<0.001). (B) Percent body fat (r=0.534, P<0.001). (C) Skeletal muscle mass (r=0.503, P<0.001).
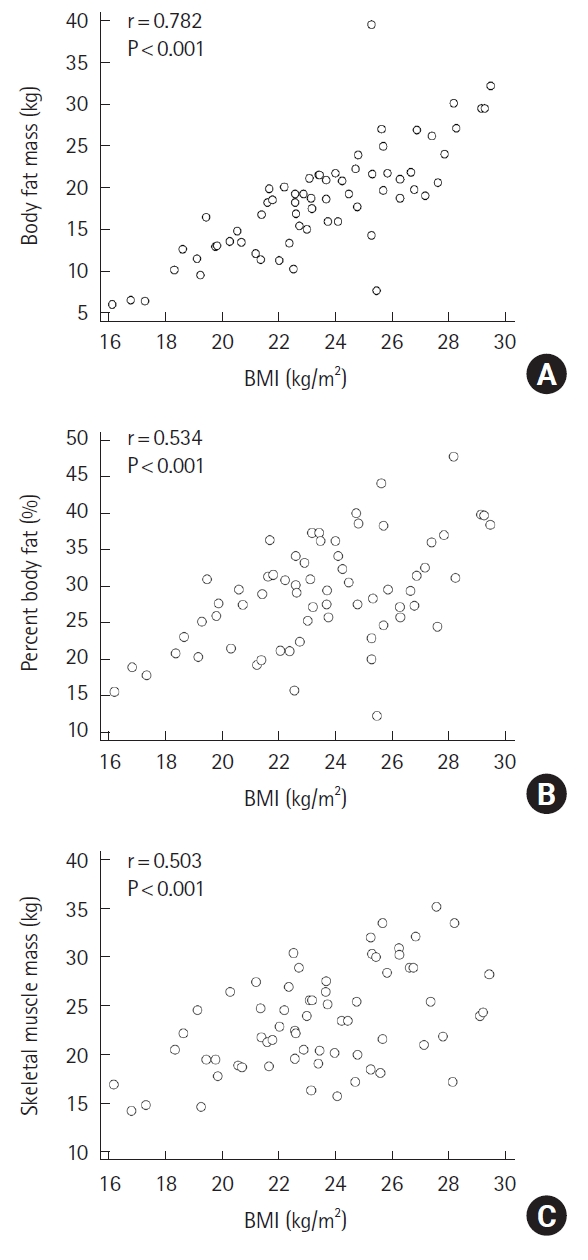
Fig. 3.
Receiver operating characteristic (ROC) curves of body fat mass and body mass index for prolonged operative time. (A) Body fat mass. Robotic surgery: area under the ROC curve (AUC), 0.800; 95% confidence interval (CI), 0.577–0.938. Laparoscopy: AUC, 0.642; 95% CI, 0.484–0.781; P=0.240. (B) Body mass index. Robotic surgery: AUC, 0.519; 95% CI, 0.330–0.704. Laparoscopy: AUC, 0.642, 95% CI, 0.484–0.781; P=0.476.
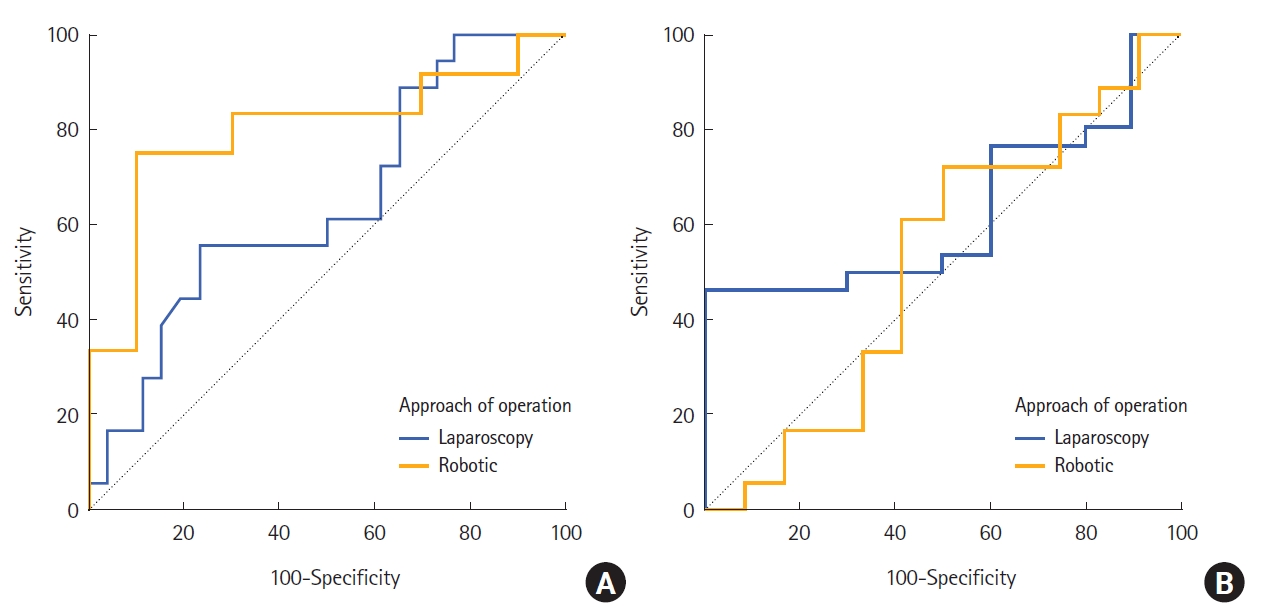
Table 1.
Patient characteristics and short-term outcomes according to the BMI
| Characteristic | BMI-low group | BMI-high group | P-value |
|---|---|---|---|
| No. of patients | 44 | 22 | |
| Age (yr) | 65.5±9.6 | 66.1±11.5 | 0.826 |
| Sex | 0.189 | ||
| Male | 21 (47.7) | 15 (68.2) | |
| Female | 23 (52.3) | 7 (31.8) | |
| BMI (kg/m²) | 21.8±2.2 | 26.9±1.4 | <0.001 |
| Body fat mass (kg) | 15.9±4.6 | 23.7±6.6 | <0.001 |
| Percent body fat (%) | 27.9±6.5 | 31.3±8.4 | 0.072 |
| Skeletal muscle mass (kg) | 2.8±1.0 | 2.9±1.2 | 0.803 |
| ASA classification | >0.999 | ||
| II | 33 (75.0) | 17 (77.3) | |
| III | 11 (25.0) | 5 (22.7) | |
| ECOG performance status | >0.999 | ||
| 0 | 37 (84.1) | 19 (86.4) | |
| 1 | 6 (13.6) | 3 (13.6) | |
| 2 | 1 (2.3) | 0 (0) | |
| Smoking | 0.392 | ||
| No | 41 (93.2) | 19 (86.4) | |
| Yes | 3 (6.8) | 3 (13.6) | |
| Prior abdominal surgery | 0.159 | ||
| No | 39 (88.6) | 16 (72.7) | |
| Yes | 5 (11.4) | 6 (27.3) | |
| Tumor location | 0.285 | ||
| Right colon | 14 (31.8) | 11 (50.0) | |
| Left colon | 24 (54.5) | 10 (45.5) | |
| Rectum | 6 (13.6) | 1 (4.5) | |
| Surgical approach | 0.583 | ||
| Laparoscopy | 28 (63.6) | 16 (72.7) | |
| Robotic surgery | 16 (36.4) | 6 (27.3) | |
| Operative method | 0.487 | ||
| Right hemicolectomy | 12 (27.3) | 10 (45.5) | |
| Transverse colectomy | 1 (2.3) | 0 (0) | |
| Left hemicolectomy | 4 (9.1) | 3 (13.6) | |
| Anterior resection | 17 (38.6) | 5 (22.7) | |
| Low anterior resection | 10 (22.7) | 4 (18.2) | |
| T categorya | 0.159 | ||
| Tis | 6 (14.0) | 1 (5.3) | |
| T1 | 8 (18.6) | 7 (36.8) | |
| T2 | 4 (9.3) | 1 (5.3) | |
| T3 | 20 (46.5) | 10 (52.6) | |
| T4 | 5 (11.6) | 0 (0) | |
| N categorya | 0.541 | ||
| 0 | 27 (62.8) | 11 (57.9) | |
| 1 | 13 (30.2) | 5 (26.3) | |
| 2 | 3 (7.0) | 3 (15.8) | |
| M categorya | 0.665 | ||
| 0 | 39 (90.7) | 16 (84.2) | |
| 1 | 4 (9.3) | 3 (15.8) | |
| Differentiationa | 0.872 | ||
| Well | 8 (18.6) | 2 (10.5) | |
| Moderate | 30 (69.8) | 15 (79.0) | |
| Poor | 4 (9.3) | 2 (10.5) | |
| Mucinous | 1 (2.3) | 0 (0) | |
| No. of harvested LNs | 21 (15.25–28.75) | 18 (15–25) | 0.292 |
| Operative time (min) | 213.6±46.7 | 231.8±48.5 | 0.146 |
| Blood loss (mL) | 50 (45–70) | 50 (50–70) | 0.867 |
| Time to diet (day) | 3.0 (3.0–3.5) | 3.0 (3.0–3.0) | >0.999 |
| Time to gas passing (day) | 3.0 (2.0–3.0) | 3 (2.0–3.0) | 0.803 |
| Postoperative hospital stay (day) | 7.0 (7.0–8.5) | 7.0 (6.0–8.0) | 0.568 |
| Morbidity | 12 (27.3) | 7 (31.8) | 0.776 |
| Clostridium difficile infection | 2 | 0 | |
| Chyle drainage | 1 | 4 | |
| Ileus | 3 | 3 | |
| Major leakage | 1 | 0 | |
| Pneumonia | 1 | 0 | |
| Surgical site infection | 2 | 0 | |
| Voiding difficulty | 2 | 0 | |
| Clavian-Dindo classificationb | 0.652 | ||
| I | 6 (50.0) | 3 (42.9) | |
| II | 5 (41.7) | 4 (57.1) | |
| III | 1 (8.3) | 0 (0) | |
| Readmission | 1 (2.3) | 1 (4.5) | >0.999 |
Table 2.
Relationship between body mass index, body composition index, and complications
Table 3.
Univariate and multivariate analyses of factors associated with prolonged operative time
Table 4.
Subgroup analysis according to tumor location, N category, and surgical approach between body mass index, body composition index, and prolonged operative time with correlation analysis
Table 5.
Predictive significance between factors, including body mass index and prolonged operative time, with the area under the curve analysis
| Variable | Area | 95% CI | P-value |
|---|---|---|---|
| Body mass index | 0.624 | 0.488–0.759 | 0.086 |
| Body fat mass | 0.662 | 0.531–0.794 | 0.024 |
| Percent body fat | 0.606 | 0.466–0.746 | 0.140 |
| Skeletal muscle mass | 0.557 | 0.417–0.697 | 0.428 |
REFERENCES
1. Kang J, Baek SE, Kim T, Hur H, Min BS, Lim JS, et al. Impact of fat obesity on laparoscopic total mesorectal excision: more reliable indicator than body mass index. Int J Colorectal Dis 2012;27:497–505.



2. Senagore AJ, Delaney CP, Madboulay K, Brady KM, Fazio VW. Laparoscopic colectomy in obese and nonobese patients. J Gastrointest Surg 2003;7:558–61.


3. Pikarsky AJ, Saida Y, Yamaguchi T, Martinez S, Chen W, Weiss EG, et al. Is obesity a high-risk factor for laparoscopic colorectal surgery? Surg Endosc 2002;16:855–8.



4. Hotouras A, Ribas Y, Zakeri SA, Nunes QM, Murphy J, Bhan C, et al. The influence of obesity and body mass index on the outcome of laparoscopic colorectal surgery: a systematic literature review. Colorectal Dis 2016;18:O337–66.



5. Tuech JJ, Regenet N, Hennekinne S, Pessaux P, Bergamaschi R, Arnaud JP. Laparoscopic colectomy for sigmoid diverticulitis in obese and nonobese patients: a prospective comparative study. Surg Endosc 2001;15:1427–30.



6. Makino T, Trencheva K, Shukla PJ, Rubino F, Zhuo C, Pavoor RS, et al. The influence of obesity on short- and long-term outcomes after laparoscopic surgery for colon cancer: a case-matched study of 152 patients. Surgery 2014;156:661–8.


7. Kamoun S, Alves A, Bretagnol F, Lefevre JH, Valleur P, Panis Y. Outcomes of laparoscopic colorectal surgery in obese and nonobese patients: a case-matched study of 180 patients. Am J Surg 2009;198:450–5.


8. Miyamoto Y, Ishii T, Tashiro J, Satoh T, Watanabe M, Baba H, et al. Effects of obesity on the outcome of laparoscopic surgery for colorectal cancer. Surg Today 2014;44:1293–9.



9. Xia X, Huang C, Jiang T, Cen G, Cao J, Huang K, et al. Is laparoscopic colorectal cancer surgery associated with an increased risk in obese patients? A retrospective study from China. World J Surg Oncol 2014;12:184.




10. Mori S, Baba K, Yanagi M, Kita Y, Yanagita S, Uchikado Y, et al. Laparoscopic complete mesocolic excision with radical lymph node dissection along the surgical trunk for right colon cancer. Surg Endosc 2015;29:34–40.



11. Watanabe J, Tatsumi K, Ota M, Suwa Y, Suzuki S, Watanabe A, et al. The impact of visceral obesity on surgical outcomes of laparoscopic surgery for colon cancer. Int J Colorectal Dis 2014;29:343–51.



12. Krane MK, Allaix ME, Zoccali M, Umanskiy K, Rubin MA, Villa A, et al. Does morbid obesity change outcomes after laparoscopic surgery for inflammatory bowel disease? Review of 626 consecutive cases. J Am Coll Surg 2013;216:986–96.


13. Mustain WC, Davenport DL, Hourigan JS, Vargas HD. Obesity and laparoscopic colectomy: outcomes from the ACS-NSQIP database. Dis Colon Rectum 2012;55:429–35.


14. Poulsen M, Ovesen H. Is laparoscopic colorectal cancer surgery in obese patients associated with an increased risk? Short-term results from a single center study of 425 patients. J Gastrointest Surg 2012;16:1554–8.



15. Akiyoshi T, Ueno M, Fukunaga Y, Nagayama S, Fujimoto Y, Konishi T, et al. Effect of body mass index on short-term outcomes of patients undergoing laparoscopic resection for colorectal cancer: a single institution experience in Japan. Surg Laparosc Endosc Percutan Tech 2011;21:409–14.


16. Park JW, Lim SW, Choi HS, Jeong SY, Oh JH, Lim SB. The impact of obesity on outcomes of laparoscopic surgery for colorectal cancer in Asians. Surg Endosc 2010;24:1679–85.



17. Bège T, Lelong B, Francon D, Turrini O, Guiramand J, Delpero JR. Impact of obesity on short-term results of laparoscopic rectal cancer resection. Surg Endosc 2009;23:1460–4.



18. Scheidbach H, Benedix F, Hügel O, Kose D, Köckerling F, Lippert H. Laparoscopic approach to colorectal procedures in the obese patient: risk factor or benefit? Obes Surg 2008;18:66–70.



19. Tsujinaka S, Konishi F, Kawamura YJ, Saito M, Tajima N, Tanaka O, et al. Visceral obesity predicts surgical outcomes after laparoscopic colectomy for sigmoid colon cancer. Dis Colon Rectum 2008;51:1757–67.


20. Rullier E, Sa Cunha A, Couderc P, Rullier A, Gontier R, Saric J. Laparoscopic intersphincteric resection with coloplasty and coloanal anastomosis for mid and low rectal cancer. Br J Surg 2003;90:445–51.



21. World Health Organization (WHO). Obesity: preventing and managing the global epidemic: report of a WHO consultation. World Health Organ Tech Rep Ser 2000;894:i–xii, 1–253.

22. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63.


23. Examination Committee of Criteria for ‘Obesity Disease’ in Japan; Japan Society for the Study of Obesity. New criteria for ‘obesity disease’ in Japan. Circ J 2002;66:987–92.


24. Seki Y, Ohue M, Sekimoto M, Takiguchi S, Takemasa I, Ikeda M, et al. Evaluation of the technical difficulty performing laparoscopic resection of a rectosigmoid carcinoma: visceral fat reflects technical difficulty more accurately than body mass index. Surg Endosc 2007;21:929–34.



25. Ishii Y, Hasegawa H, Nishibori H, Watanabe M, Kitajima M. Impact of visceral obesity on surgical outcome after laparoscopic surgery for rectal cancer. Br J Surg 2005;92:1261–2.



26. Kim SS, Kim JH, Jeong WK, Lee J, Kim YK, Choi D, et al. Semiautomatic software for measurement of abdominal muscle and adipose areas using computed tomography: a STROBE-compliant article. Medicine (Baltimore) 2019;98:e15867.



28. Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation: technical notes and outcome. Colorectal Dis 2009;11:354–65.


29. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13.



30. He Q, Horlick M, Thornton J, Wang J, Pierson RN Jr, Heshka S, et al. Sex and race differences in fat distribution among Asian, African-American, and Caucasian prepubertal children. J Clin Endocrinol Metab 2002;87:2164–70.


31. Böhm A, Heitmann BL. The use of bioelectrical impedance analysis for body composition in epidemiological studies. Eur J Clin Nutr 2013;67 Suppl 1:S79–85.


32. Kim EY, Kim SR, Won DD, Choi MH, Lee IK. Multifrequency bioelectrical impedance analysis compared with computed tomography for assessment of skeletal muscle mass in primary colorectal malignancy: a predictor of short-term outcome after surgery. Nutr Clin Pract 2020;35:664–74.



33. Sergi G, De Rui M, Stubbs B, Veronese N, Manzato E. Measurement of lean body mass using bioelectrical impedance analysis: a consideration of the pros and cons. Aging Clin Exp Res 2017;29:591–7.



34. Malbrain ML, Huygh J, Dabrowski W, De Waele JJ, Staelens A, Wauters J. The use of bio-electrical impedance analysis (BIA) to guide fluid management, resuscitation and deresuscitation in critically ill patients: a bench-to-bedside review. Anaesthesiol Intensive Ther 2014;46:381–91.


35. Kumar S, Dutt A, Hemraj S, Bhat S, Manipadybhima B. Phase angle measurement in healthy human subjects through bio-impedance analysis. Iran J Basic Med Sci 2012;15:1180–4.


36. Lukaski HC, Kyle UG, Kondrup J. Assessment of adult malnutrition and prognosis with bioelectrical impedance analysis: phase angle and impedance ratio. Curr Opin Clin Nutr Metab Care 2017;20:330–9.


37. Buffa R, Mereu E, Comandini O, Ibanez ME, Marini E. Bioelectrical impedance vector analysis (BIVA) for the assessment of two-compartment body composition. Eur J Clin Nutr 2014;68:1234–40.



- TOOLS