Multimodal prerehabilitation for elderly patients with sarcopenia in colorectal surgery
Article information
Abstract
Sarcopenia, which is characterized by progressive and generalized loss of skeletal muscle mass and strength, has been well described to be associated with numerous poor postoperative outcomes, such as increased perioperative mortality, postoperative sepsis, prolonged length of stay, increased cost of care, decreased functional outcome, and poorer oncological outcomes in cancer surgery. Multimodal prehabilitation, as a concept that involves boosting and optimizing the preoperative condition of a patient prior to the upcoming stressors of a surgical procedure, has the purported benefits of reversing the effects of sarcopenia, shortening hospitalization, improving the rate of return to bowel activity, reducing the costs of hospitalization, and improving quality of life. This review aims to present the current literature surrounding the concept of sarcopenia, its implications pertaining to colorectal cancer and surgery, a summary of studied multimodal prehabilitation interventions, and potential future advances in the management of sarcopenia.
INTRODUCTION
Sarcopenia is a progressive and generalized disorder of muscle atrophy that is primarily associated with aging, but has also been observed in even younger populations (as early as 30 years) secondary to nutritional deficits, physical immobility, and systemic disease, such as malignancy. Evidence has demonstrated the global prevalence of sarcopenia to be between 10% and 27% in individuals aged ≥60 years [1]. With advances in medicine and overall life expectancy, sarcopenia has been emerging as a significant modifiable risk factor for adverse outcomes in surgical candidates. Specific to colorectal surgery, sarcopenia has implications for early and long-term postoperative outcomes, functional outcomes, and oncological outcomes.
Despite the introduction of Enhanced Recovery After Surgery (ERAS) protocols nearly 2 decades ago, the safety and efficacy of which in colorectal surgery have been observed in large multinational multicenter trials and meta-analyses, complications after colorectal surgery can occur in as many as 45% of patients. Multimodal prehabilitation, as a complement to the perioperative measures of ERAS protocols, offers a potential treatment modality to help circumvent the negative effects of sarcopenia, prepare the patient for surgical stress, and promote earlier recovery [2].
This review aims to present the current understanding of sarcopenia, the effects of sarcopenia on colorectal surgery, potential prehabilitation interventions, and potential future advances in the management of sarcopenia in patients undergoing colorectal surgery.
DEFINING SARCOPENIA
In the last decade, the International Working Group on Sarcopenia (IWGS) [3], Foundation for the National Institutes of Health (FNIH) [4], Asian Working Group for Sarcopenia (AWGS) [5], and European Working Group on Sarcopenia in Older People (EWGSOP) [6] have established international standards on sarcopenia. The consensus definition of sarcopenia comprises 3 parameters: (1) loss of skeletal muscle mass along with (2) loss of muscle strength and/or (3) reduced physical performance. The presence of all 3 parameters is consistent with severe sarcopenia.
To date, no single diagnostic criterion for sarcopenia has been established, mostly due to the heterogeneity of this disease. Instead, attempts have instead centered on delineating tailored population-based cutoffs. According to the AWGS 2019 update [5], sarcopenia in Asian men is defined as muscle mass <7.0 kg/m2 with a handgrip strength <28.0 kg and/or a gait speed <1.0 m/sec. In women, it is defined as muscle mass <5.4 kg/m2 via dual-energy x-ray absorptiometry (DXA) or <5.7 kg/m2 via bioelectrical impedance analysis (BIA), with a handgrip strength <18.0 kg and/or gait speed <1.0 m/sec.
DXA has high reproducibility but is time-consuming and therefore mainly used in research. In contrast, BIA is quick and inexpensive with reasonable reproducibility and can be used easily for screening. Clinically, because most surgical patients undergo preoperative computed tomography (CT) imaging for diagnosis and operative planning, observational studies have primarily utilized CT scans to define sarcopenia based on low muscle mass. This has been shown to be a useful imaging biomarker for predicting preoperative nutritional risk in elderly colorectal cancer patients [7]. The skeletal muscle index at the level of L3 is the most common measure; however, other parameters, including the psoas muscle area at L4 and dorsal muscle group area at T12, have also been used to diagnose sarcopenia [8]. Muscle mass dimensions on ultrasonography [9] and magnetic resonance imaging [10] are also similarly reproducible and reliable, representing alternative modalities to CT imaging.
Sarcopenia is prevalent in 10% of general elderly patients worldwide [11], in 14.7% of hospitalized older patients [12], 41% to 59% of nursing home residents [13], and 38.6% of cancer patients [14]. While the tumor subsite is one of the major determinants of malnutrition, with pancreatic, esophageal, head and neck, and lung cancer having the highest prevalence [15], sarcopenia is seen in up to 60% of patients with colorectal cancer [16].
PATHOPHYSIOLOGY OF SARCOPENIA
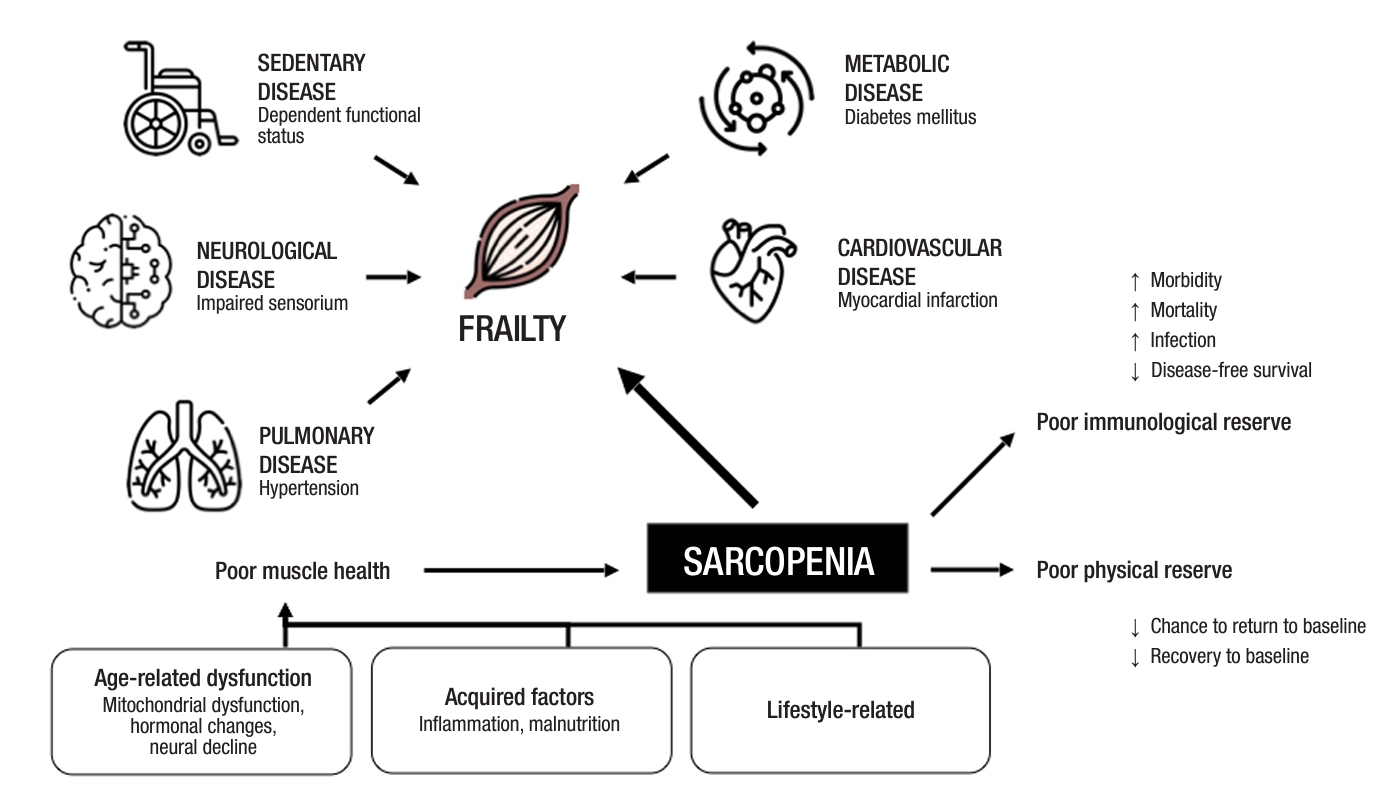
Multiple metabolic processes contribute to the development of sarcopenia over time (Fig. 1). Intrinsic, or nonmodifiable factors include age-related mitochondrial dysfunction and the waning of muscle stem cells’ ability to regenerate. Aging is associated with reduced anabolic efficiency in older adults when compared to their younger counterparts in response to amino acid and glucose-rich food [17], which results in less protein synthesis despite similar diets. This may be mitigated with amino acid or protein supplementation to stimulate protein anabolism, as well as physical activity, which sensitizes aging muscle to subsequent nutritional stimuli [17].
Recent evidence has also linked age-related obesity to the pathogenesis of sarcopenia. As adipose tissue redistributes to the visceral area and infiltrates into muscle with age, it results in local myosteatosis, inducing mitochondrial dysfunction, reduction in β-oxidation of fatty acids, and production of reactive oxygen species [18]. This triggers low-grade chronic inflammation and systemic insulin resistance via proinflammatory cytokines and chemokines, resulting in the suppression of protein synthesis, stimulation of protein catabolism, and muscle atrophy [19]. Overall, these mechanisms cause an impaired balance between protein synthesis and proteolysis, yielding sarcopenia.
Extrinsic factors include lifestyle factors such as prolonged bed rest, surgical stress, malnutrition, or chronic disease. These processes are further intensified in the perioperative period, when patients undergoing gastrointestinal surgery typically fasted and sedentary for prolonged recovery periods, decelerating perioperative protein synthesis. Surgery also provokes a proinflammatory response that exacerbates muscle atrophy. It is therefore imperative to combat preoperative sarcopenia, which affects a myriad of adverse postoperative outcomes.
ADVERSE POSTOPERATIVE OUTCOMES IN SARCOPENIA IN COLORECTAL SURGERY
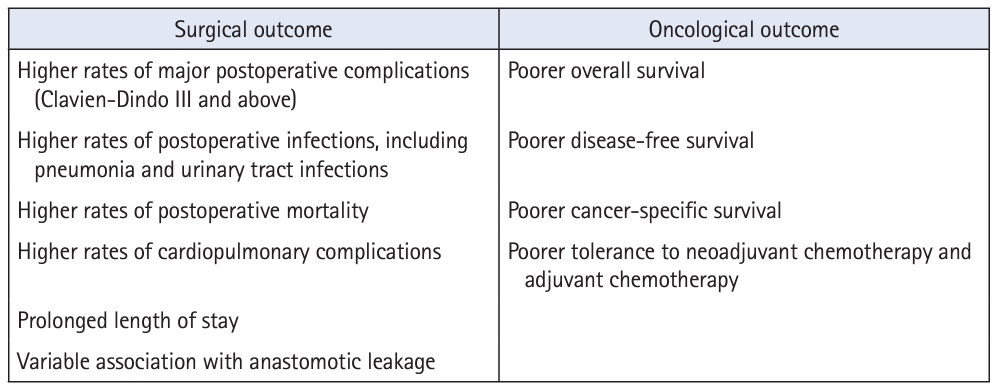
A summary of the impacts of sarcopenia on surgical and oncological outcomes is detailed in Fig. 2.
Surgical outcomes
Sarcopenia is an independent predictor of poor surgical outcomes. It is associated with higher postoperative mortality in emergency general surgery [20–24], abdominal trauma [25], gastric cancer [26], hepatocellular carcinoma [27], pancreatic cancer [28], and biliary cancer [29]—and colorectal cancer is no exception. A meta-analysis of 44 observational studies comprising 18,891 patients with colorectal cancer conducted by Trejo-Avila et al. [30] revealed an association between sarcopenia and a higher risk of total postoperative complications, including infections and cardiopulmonary complications. Sarcopenia was also found to be significantly correlated with general postoperative infections at locations other than surgical sites, such as pneumonia and urinary tract infections [31]. A possible mechanism for occurrence is that sarcopenia causes an increased postoperative inflammatory response, as evidenced by significantly higher plasma concentrations of inflammatory markers such as calprotectin [32] and the neutrophil to lymphocyte ratio [33]. Amongst all postoperative complications, most studies on anastomotic leakage showed no significant difference in occurrence according to the presence or absence of sarcopenia [30, 31] except for 1 study [34] that found psoas density to be an independent predictor for anastomotic leakage. The heterogeneity of outcomes in the literature is largely attributable to variations in the definitions of sarcopenia used by publications and the type of measurements they have utilized, highlighting that standardized diagnostic methods should be adopted.
Oncological outcomes
Sarcopenia has been associated with incomplete neoadjuvant treatment and poor tolerance to postoperative chemotherapy, with resultant effects on prognosis and overall survival [35]. Reduced muscle mass renders patients more susceptible to toxic effects during chemotherapy and thereby reduces treatment efficacy, possibly attributable to alterations in the pharmacokinetics of the drugs [36].
Several meta-analyses have also demonstrated that patients with sarcopenia have significantly shorter overall survival, disease-free survival, and cancer-specific survival than patients without sarcopenia [7, 30]. These results emphasize the crucial role of the early detection and prevention of sarcopenia in patients with colorectal cancer.
The mechanisms underlying unfavorable oncological outcomes in patients with sarcopenia are poorly understood. One hypothesis hints at an immunological impact associated with poor muscle health. Skeletal muscle has been shown to be associated with tumor‐infiltrating lymphocytes (TILs) in patients with colorectal cancer, where increased TILs (e.g., CD3+, CD8+, CD4+, and FOXP3+ T cells) may not only provide strong host immune reactions against cancer cell proliferation [37] but also effectively predict response to chemotherapy [38]. This mechanism is not unique to colorectal cancer, and these clinical findings have been replicated in gastric cancer, gynecological malignancies, and hepatobiliary cancers.
POTENTIAL INTERVENTIONS
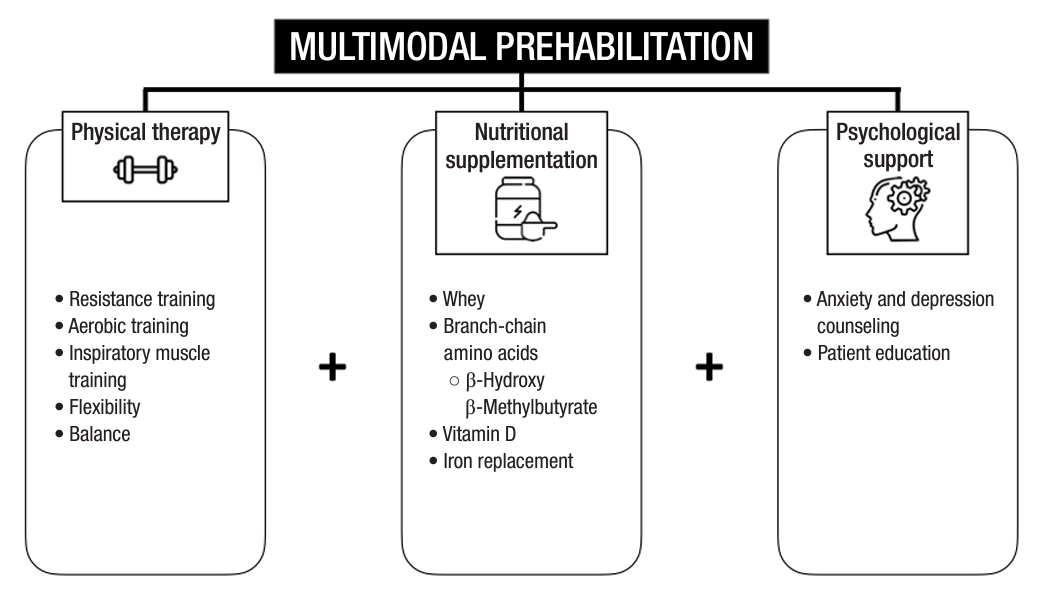
The concept of prehabilitation is based on the notion that the presurgical period is a “window of opportunity” to boost and optimize the premorbid nutrition of a patient, providing a compensatory “buffer” of physiological reserve for the impending insult of a surgical procedure [39]. This can be achieved through a multimodal prehabilitation program comprising nutrition, exercise, patient education, a comprehensive geriatric assessment, and quality of life assessments to improve patients’ physical fitness and capacity, optimize nutritional status, and promote psychological resilience (Fig. 3). Careful patient screening and selection to identify sarcopenic individuals preoperatively and address risk factors of poor muscle health with multimodal interventions can reduce potential perioperative adverse outcomes. A structured prehabilitation program especially for the geriatric population has been shown to shorten hospitalization, improve the rate of return to bowel activity, reduce the costs of hospitalization, and improve quality of life after surgery [40–43].
Physical therapy
Exercise and physical therapy have been demonstrated to improve physical fitness, enhance quality of life, alleviate psychological anxiety and depression, and reduce fatigue during cancer treatment [44], and have been the mainstay of unimodal prehabilitation programs for colorectal cancer patients. Exercise modalities include aerobic training, resistance training, flexibility, and balance.
Resistance training, in particular, is known to promote muscle hypertrophy and increase muscle mass, strength, and function [45], and can be widely adopted even in frail elderly patients. These measures have objectively showed improvements in peak oxygen uptake [46], daily step count [47], and 6-minute walking test scores [48], which have translatable effects on the length of stay, readmission rates, tolerance to neoadjuvant treatment, and postoperative outcomes [44]. Exercise interventions also have impacts beyond physical fitness, showing improvements in depression and anxiety symptoms and quality of life scores [49].
Nutritional supplementation
Preoperative nutritional supplementation for at-risk individuals can increase preoperative muscle mass and strength. The potential for postoperative oral nutrition for postdischarge patients after colorectal cancer surgery in reducing sarcopenia prevalence has also been demonstrated in a randomized controlled trial showing a higher skeletal muscle index in patients who received oral nutritional supplementation than in those who received dietary advice alone [50].
The optimal type and regimen for oral supplementation for sarcopenia and the improvement of muscle strength remain unknown, possibly due to the fact that nutritional supplementation should not be a “one-size-fit-all” strategy, but rather one that is personalized and tailored to the metabolic needs and demands of different patients. Nevertheless, various schools of thought exist regarding the type of nutritional supplementation required for perioperative patients. For muscle synthesis and anabolism, protein supplementation has been the mainstay of nutritional prehabilitation in many studies. One common form of protein is whey protein, which is extracted from cow’s milk, containing all 9 essential amino acids required for anabolic processes. Whey protein also contains high amounts of cysteine, which contributes to the synthesis of intracellular glutathione, a dietary oxidant that has been shown to reduce oxidative stress in sarcopenic patients, especially under surgical stress [51]. Other studies have evaluated the effect of branched-chain amino acids, constituting leucine, isoleucine, and valine, which have shown positive impacts on muscle mass and strength [52]. β-Hydroxy-β-methylbutyrate (HMB), an active metabolite of leucine, which has well-established impacts on muscle strength in the elderly population [53], is being evaluated for similar efficacy in the preoperative prehabilitative setting. An ongoing study in Singapore, in partnership with Abbott Nutrition—the HEROS (Oral Nutritional Supplementation With HMB Enhance Muscle Quality in Sarcopenic Surgical Patients) study—has set out to evaluate the effects of HMB supplementation on intramuscular adipose tissue in the setting of multimodal prehabilitation for major gastrointestinal surgery patients [54].
Other nutrients also play a role in supplementing the anabolic response, and vitamin D is widely known to have benefits for muscle contraction, energy metabolism, and function via its effects on protein synthesis, skeletal muscle hypertrophy, and regulation of mitochondrial oxidative phosphorylation [55]. Preoperative intravenous iron supplementation and correction of iron deficiency anemia have been found to significantly reduce postoperative complications and the need for postoperative transfusions, which negatively impact disease-free survival [56].
Psychological support and counseling
It is expected that patients who have been diagnosed with colorectal cancer face a significant amount of stress, depression, and anxiety, which have direct effects on poorer surgical outcomes, including heightened pain sensitivity [57] and impaired wound healing from glucocorticoid production. The prevalence of depression and anxiety among colorectal cancer patients is as high as 57% and 47%, respectively [58], emphasizing the importance of early identification and treatment. Patients can be screened using validated questionnaires, such as the Generalized Anxiety Disorder-7 (GAD-7) and Patient Health Questionnaire-9 (PHQ-9). Several studies have attempted to provide psychological support in the form of breathing and relaxation exercises, as well as cognitive behavioral therapy.
The synergistic effects of nutritional therapy, physical exercise and psychological support have been well demonstrated; not only do dietary and physical activity interventions have positive effects on quality of life [59], but an improved outlook on life also affects compliance with treatment and therapeutics, thus underscoring the importance of a multimodal approach.
Compliance is an important element of prehabilitation. For use in practice, the tools employed need to be cost-effective, standardized, and repeatable by practitioners in a variety of clinical settings and across different patient populations. Targeting patients at risk, combining protein supplements with strength training, and defining standardized patient-related outcomes will be essential for obtaining satisfactory results [60].
Sustained treatment for sarcopenia seems to confer benefits even postoperatively, as seen in a retrospective cohort study by Lee et al. [61] involving 2,333 patients, where both overall survival and recurrence-free survival were lower in patients with persistent sarcopenia 2 to 3 years postoperatively than in those who recovered (overall survival: 96.2% vs. 90.2%, P=0.001; recurrence-free survival: 91.1% vs. 83.9%, P=0.002).
Screening patients for a prehabilitation program
The indiscriminate inclusion of patients with colorectal cancer in prehabilitation programs may incur excessive healthcare costs and utilization of resources. In order to maximize benefits from a curated multidisciplinary prehabilitation program, resources should be targeted at patient groups who require it the most, such as those with sarcopenia and frailty. To date, no study has conclusively demonstrated the cost-effectiveness of such initiatives.
High-risk individuals who are elderly, frail, or manifest risk factors for sarcopenia, such as the presence of chronic conditions (heart failure, chronic obstructive pulmonary disease, diabetes mellitus, chronic kidney disease), functional decline, unintentional weight loss, repeated falls, and malnutrition [62], should be selected for objective measurements of sarcopenia based on muscle strength, physical performance, or skeletal muscle mass. While screening tools such as the SARC-F (Strength, Assistance walking, Rising from a chair, Climbing stairs, and Falls) questionnaire [63] exist for sarcopenia in the elderly, these tools have low sensitivity and have yet to be validated against CT-diagnosed sarcopenia [64]. CT-defined sarcopenia and myosteatosis are prevalent in preoperative colorectal cancer patients regardless of the presence of traditional nutritional risk factors (weight loss and problems eating); therefore, CT image analysis effectively adds value to nutrition screening by identifying patients with other risk factors for poor outcomes [65].
At the authors’ institution, a philanthropically sponsored prehabilitation program was initiated for patients 70 years old and above scheduled to undergo major colectomies [40]. These interventions included the following: (1) 3 weeks of oral nutrition supplementation recommended by the dietetics team; (2) 3 weeks of resistance exercises using resistance bands with weekly physiotherapist review; (3) a geriatrician consultation to optimize polypharmacy and other comorbidities among the “geriatric giants”; and (4) a thorough cardiovascular consult including preoperative transthoracic echocardiography to optimize preoperative cardiac risk. A comparison with patients with similar baseline characteristics who did not undergo the program showed a significantly shorter length of hospitalization, improvement in quality of life (based on the EuroQol-5 dimension score), and reduced costs, although anthropometric and functional characteristics, as well as morbidity rates, remained similar. The relatively short duration of the intervention could be a plausible explanation accounting for the absence of observed differences regarding measurable physical and functional attributes prior to surgery, which have been observed in the other studies mentioned above.
LIMITATIONS OF CURRENT LITERATURE
Present studies on prehabilitation in colorectal surgery are very heterogeneous in terms of the interventions and measured outcomes, hindering the standardization and adoption of this approach in clinical practice [44]. There are currently no standardized multimodal prehabilitation programs available, although efforts are being made to design and test formulated interventions through ongoing randomized controlled trials [66, 67]. A multimodal program inevitably requires an orchestrated effort and mobilization of resources from various disciplines, including physicians, surgeons, therapists, and nutritionists, and the cost-effectiveness of such an approach will need to be further evaluated.
Another major limitation is that the currently available studies on prehabilitation have mostly recruited patients using a “one-size-fits-all” approach, which may skew the results to show little or no significant effect. A selective approach to targeting only high-risk patients, who will reap more benefits from such programs, may allow us to observe better outcomes.
FUTURE ADVANCEMENTS
While current treatment strategies for sarcopenia focus on lifestyle modification and nutritional supplementation, these therapies have limited benefit to those who are immobile or unable to tolerate preoperative enteral nutrition. Novel therapeutics through the identification of targetable regions for cellular interventions are being evaluated as potential future treatments of sarcopenia. Multiomics profiling studies have been conducted to establish a comprehensive understanding of molecular changes and signaling networks involved in the pathogenesis of sarcopenia, which may lead to new clinical applications [68]. The use of regenerative medicine and stem cell therapy, which have mitochondrial restoration effects and immune modulatory abilities, offers potential for new promising therapies that may enable sarcopenia treatment on a molecular level [69].
At the same time, new modalities of assessing sarcopenia have been under development, with devices utilizing point-of-care ultrasound technology to measure intramuscular adipose tissue as a rapid assessment tool [70]. This potentially enables interval assessments of sarcopenia to track patients’ functional changes at regular intervals to monitor the efficacy of the prehabilitation program.
It is foreseeable that a multimodal, multidisciplinary approach modulated to suit an individual’s unique clinical, lifestyle, and molecular profile can be employed to combat this increasing health problem in our aging population.
CONCLUSION
Sarcopenia is prevalent in the aging population and negatively impacts surgical and oncological outcomes, as well as quality of life. Multimodal prehabilitation might be the way forward for improving outcomes, with promising data in selected groups of patients. Deeper scientific studies with close translation to clinical practice coupled with industrial collaboration can further refine the management of sarcopenia.
Notes
Conflict of interest
Frederick H. Koh is an Editorial Board member of Annals of Coloproctology, but was not involved in the reviewing or decision process of this manuscript. No other potential conflict of interest relevant to this article was reported.
Funding
None.
Author contributions
Conceptualization: FHK; Formal analysis: all authors; Visualization: FHK; Writing–original draft: JW, HC; Writing–review & editing: all authors. All authors read and approved the final manuscript.