Female Sex and Right-Sided Tumor Location Are Poor Prognostic Factors for Patients With Stage III Colon Cancer After a Curative Resection
Article information
Abstract
Purpose
Stage-IIIC colon cancer is an advanced disease; however, its oncologic outcomes and prognostic factors remain unclear. In this study, we aimed to determine the predictors of disease-free survival (DFS) in patients with stage-IIIC colon cancer.
Methods
From a multicenter database, we retrospectively enrolled 611 patients (355 men and 256 women) who had undergone a potentially curative resection for a stage-IIIC colon adenocarcinoma between 2003 and 2011. The primary end-point was the 5-year DFS.
Results
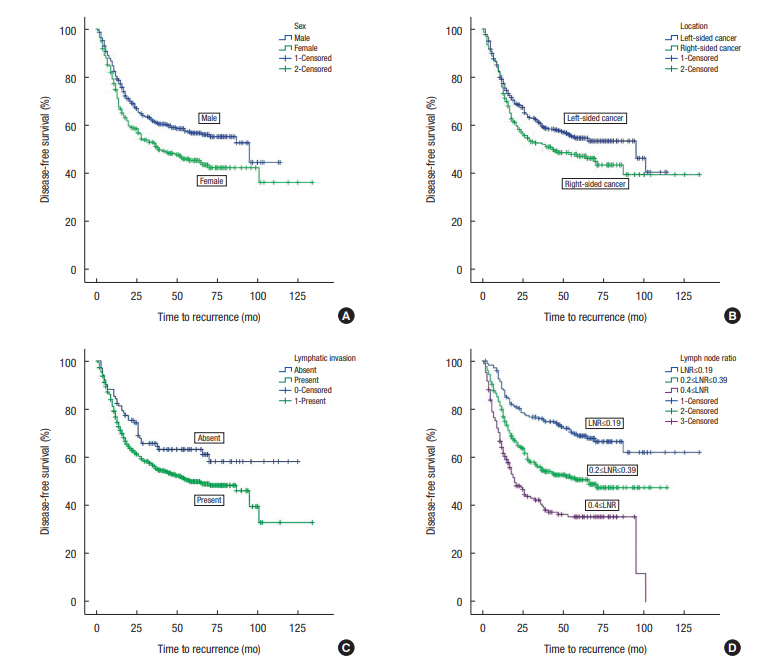
The median age was 62 years; 213 and 398 patients had right-sided colon cancer (RCC) and left-sided colon cancer (LCC), respectively. The 5-year DFS in all patients was 52.0%; median follow-up time was 35 months (range, 1–134 months). A multivariate Cox regression revealed that female sex (hazard ratio [HR], 1.50; 95% confidence interval [CI], 1.19–1.90; P < 0.01), right-sided tumor location (HR, 1.65; 95% CI, 1.29–2.11; P < 0.01), lymphatic invasion (HR, 1.52; 95% CI, 1.08–2.15; P < 0.01) and a high (≥0.4) metastatic lymph node ratio (HR, 3.72; 95% CI, 2.63–5.24; P < 0.01) were independent predictors of worse 5-year DFS. Female patients with RCC were 1.79 fold more likely to experience recurrence than male patients with LCC.
Conclusion
Female sex and right-sided tumor location are associated with higher tumor recurrence rates in patients with stage-IIIC colon cancers. Aggressive treatment and close surveillance should be planned for patients in these groups.
INTRODUCTION
Colorectal cancer is one of the most common cancers worldwide. In 2014, it was the third most commonly diagnosed cancer in Korea and the most commonly diagnosed cancer among women 65 years of age and older [1]. The mainstay treatment for patients with colon cancer is surgical resection with en bloc removal of the regional lymph nodes.
The prognosis for patients with colon cancer has improved over the past several decades owing in part to early detection [2]. Despite advances in surgical techniques and adjunctive chemotherapy, patients with stage-IIIC colon cancer have poor survival rates; the 5-year survival rate is about 28.0% [3]. Because few studies investigating the prognostic factors in patients with stage-IIIC colon cancer have been reported in the literature, the aim of this study was to identify the clinicopathologic factors that influence the 5-year disease-free survival (DFS) in patients with this particular disease stage of colon cancer.
METHODS
Patients
Between 2003 and 2011, 611 patients with stage IIIC colon cancers were sequentially enrolled from 5 referral centers in Korea. All patients had been histologically diagnosed with an adenocarcinoma of the colon and had undergone potentially curative surgery. An adjuvant chemotherapy based on 5-fluorouracil was given after surgery. Patients with histology other than an adenocarcinoma; patients with rectal cancer, hereditary cancer, synchronous colon cancer, metachronous cancer or other primary cancers; patients with less than 12 lymph nodes evaluated; patients who did not receive chemotherapy and patients who died within 30 days after surgery were excluded. All data on clinical and pathological features were reviewed retrospectively. This study was approved by the Hallym University Institutional Review Board (12-I048). The Institutional Review Board agreed to waive the requirement to obtain informed written consent from each patient.
Right-sided colon cancer (RCC) was defined as a tumor arising from the appendix, cecum, ascending colon, hepatic flexure, and proximal two-thirds of the transverse colon. Tumors arising from the distal third of the transverse colon, splenic flexure, descending colon, rectosigmoid junction and sigmoid colon were defined as left-sided colon cancers (LCCs).
Specimens were examined by pathologists at each of the five centers. The TNM stage was defined according to the American Joint Committee on Cancer (7th edition) [3]. More than 12 lymph nodes were examined for each specimen. The lymph node ratio (LNR) was defined as the number of metastatic lymph nodes divided by the number of retrieved lymph nodes. DFS was defined as the time interval between the date of surgery and the date of the detection of recurrence, last follow-up, or death. Adjuvant chemotherapy with a 5-fluorouracil-based regimen was administrated. Patients’ demographics and pathological and oncologic outcomes were compared between the RCC and the LCC groups.
Statistical analyses
All statistical analyses were performed using the IBM SPSS Statistics ver. 24.0 (IBM Co., Armonk, NY, USA). Categorical variables were analyzed using the chi-square test. Analyses of continuous variables were performed using the Student t-test. The DFS was analyzed using the Kaplan-Meier method, and comparisons were performed using the log-rank test. The Cox proportional-hazards model was used for multivariate analyses to identify the prognostic factors for 5-year DFS. Statistical tests were two-sided, and a P-value less than 0.05 was considered statistically significant.
RESULTS
Patients’ characteristics
The clinicopathologic data of 611 patients are summarized in Table 1. The median age of the patients was 62 years (range, 17–93 years), and 355 patients (58.1%) were male. Of the 611 patients, 398 (65.1%) had been diagnosed with LCC. The mean tumor size was 6.24 cm, the mean number of retrieved lymph nodes was 32.5 and the LNR was 0.31.
Analysis of prognostic factors for survival
The median overall follow-up period was 35 months (range, 1–134 months). The univariate analysis showed that female sex, RCC, N2b category, LNR ≥ 0.4, and lymphatic invasion were predictors of poor DFS (Table 2). The multivariate Cox regression analysis revealed that female sex, RCC, LNR ≥ 0.4 and lymphatic invasion were associated with poor 5-year DFS (Table 3).
The 5-year DFSs were poorer for female sex versus male sex (45.5% ± 3.3% vs. 56.8% ± 2.8%, respectively), for patients with RCC than for those with LCC (47.2% ± 3.6% vs. 54.7% ± 2.7%, respectively) and for patients with lymphatic invasion versus those without (49.9% ± 2.4% vs. 63.3 ± 4.9%). The 5-year DFS rates were 68.9% ± 3.7% in the LNR ≤ 0.19 group, 50.7% ± 3.2% in the LNR = 0.2–0.39 group, and 35.4% ± 4.0% in the LNR ≥ 0.4 group (Fig. 1).
Additional analysis of the role of tumor location
As shown in Fig. 2, category T3N2b diseases were more prevalent in patients with LCC than in those with RCC (52% vs. 29%, respectively). The reverse was true for T4 tumors. Compared to patients with LCC, those with RCC had tumors with a more advanced T stage (T4: 71.4% vs. 48.0%, P < 0.01), a larger number of resected lymph nodes (mean ± standard deviation: 35.95 ± 16.09 vs. 30.67 ± 14.28, P < 0.01), and a greater frequency of lymphatic invasion (85.9% vs. 83.7%, P < 0.01). However, patients with LCC had more advanced N stage (N2: 84.1% vs. 71.2%, P < 0.01) and greater LNRs (≥0.4: 32.7% vs. 17.4%, P < 0.01).
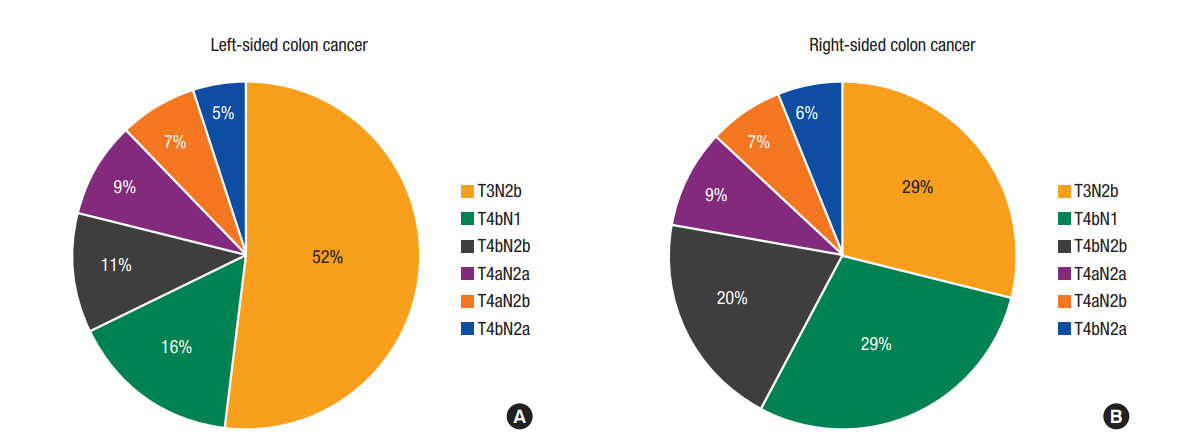
The different distributions of T and N categories in patients with left-sided colon cancer (LCC) (A) and right-sided colon cancer (RCC) (B). (A) T3N2b disease was more common among LCCs. (B) T4 category diseases were more common among RCCs.
Female patients with RCC were 1.79 fold more likely to experience recurrence than male patients with LCC (hazard ratio [HR], 1.79; 95% confidence interval [CI], 1.27–2.51, P < 0.01). No significant differences between the LCC and the RCC groups with respect to mean age, sex, tumor size, and number of metastatic lymph nodes were noted.
DISCUSSION
In this retrospective study, data on 611 patients with stage-IIIC colon cancer were extracted from a multicenter database. On multivariate analysis, we demonstrated that female sex, right-sided tumor location, lymphatic invasion and high LNR were poor prognostic factors for 5-year DFS.
It is well established that lymph node metastasis is related to adverse outcomes following a colon cancer resection. Conventionally, lymph node staging of colon cancer is based on the American Joint Committee on Cancer N category. However, several studies have suggested that the LNR could substitute for the N category [4-6]. Our data revealed a strong relationship between LNR and 5-year DFS. The LNR ≥ 0.2 and the LNR ≥ 0.4 subgroups were associated with 2.13 fold and 3.53 fold increases in the HR, respectively.
There is an ongoing debate regarding the differences in survival rates between patients with RCC and those with LCC [7]. Overall, the oncologic outcome of patients with RCC is inferior to that of patient with LCC, but this trend varies depending on the stage [8]. In a study by Meguid et al. [9], patients with stage-III RCC exhibited worse 5-year overall survival than their counterparts with LCC, as did those with stage-IV RCC. In contrast, patients with stage-II RCC showed better survival rates than their LCC counterparts. Two successive studies by Weiss et al. [10, 11] and studies performed in Asian countries [12-14] came to similar conclusions. Taken together, for early stage disease, the survival of patients with RCC is equal to or better than that of patients with LCC, but it becomes poorer as the disease progresses.
The difference between RCC and LCC is attributable to tumor biology. Many studies have reported biologic differences of RCC and LCC. Microsatellite instability (MSI) positive status is common in RCC, and 20%–25% of patients with stage-II RCC are shown to be MSI positive. MSI-positive status is less than 15% for stage-III RCC and even lower for stage-IV disease. MSI is related to lower stage RCC and is a favorable predictor of survival [10, 11]. Other than MSI status, KRAS and BRAF expressions are also associated with RCC while NRAS mutation and chromosome instability are more common in patients with LCC [15].
Another potential cause of poor outcome in the patients with LCC may be fewer lymph node dissections [16]. The surgical extent for a right hemicolectomy has been well standardized to be from the terminal ileum to half of the transverse colon [17]. However, the extent of lymph node dissection in a left hemicolectomy varies [16, 18]. Patients with LCC are associated with fewer dissected lymph nodes and a high LNR. Therefore, the standardized techniques of central vascular ligation and total mesocolic excision should be strictly observed in a left hemicolectomy. In the treatment of patients with RCC, incomplete tumor removal in the retroperitoneal resection margin could be a source of recurrence. Therefore, achieving an adequate circumferential resection margin and a complete mesocolic excision with proper dissection plane for patients with RCC is important.
This study has some inherent limitations. First, it is based on a retrospective multicenter database; hence, significant selection bias may exist. Data in this study represent uniform Korean populations; hence, the result would be different from those of previous studies using the Surveillance, Epidemiology and End Results databases. Second, the recurrence patterns of RCC and LCC were not analyzed due to loss during follow-up and insufficient data. Classifying the recurrence pattern in patients with stage-IIIC colon cancer is important for guiding treatment. Third, some important treatment-related factors were not considered. Combined resections of adjacent organ structures, such as pancreaticoduodenectomy in patients with right colon cancer with duodenal invasion, were performed in some cases. Pathologic data regarding tumor grade, MSI status, and circumferential resection margin were not available. Chemotherapeutic regimens, doses, treatment durations, and treatment failure rates were also not available. The low socioeconomic status of elderly female patients may have led to poor compliance and adverse outcome.
Despite these limitations, our study is the first to analyze patients with stage-IIIC colon cancer separately, and it produced some important findings. We confirmed that the survival of patients with stage-IIIC RCC is inferior to that of their LCC counterparts. We also found that female sex is another factor for poor prognosis for patients with stage-IIIC colon cancer. When combined, these two clinical factors could be strong predictors of poor outcome.
In conclusion, stage-IIIC colon cancer produces poor oncologic outcomes when occurring in female patients, when it is right-sided, and when the LNR is high. Therefore, a reasonable approach would be to plan more aggressive treatments for female patients with RCC; close surveillance for disease recurrence is also desirable in this group. Further clinical and genetic studies are required to elucidate the mechanisms underlying our findings and to confirm those findings in a large-scale setting.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.