Validation of Korean Version of Low Anterior Resection Syndrome Score Questionnaire
Article information
Abstract
Purpose
Patients who undergo radical surgery for rectal cancer often experience low anterior resection syndrome (LARS). Symptoms of this syndrome include frequent bowel movements, gas incontinence, fecal incontinence, fragmentation, and urgency. The aim of this study was to investigate the convergent validity, discriminative validity, and reliability of the Korean version of the LARS score questionnaire.
Methods
The English LARS score questionnaire was translated into Korean using the forward-and-back translation method. A total of 146 patients who underwent radical surgery for rectal cancer answered the Korean version of the LARS score questionnaire including an anchor question assessing the impact of bowel function. Participants answered the questionnaire once more after 2 weeks.
Results
The Korean LARS score questionnaire showed high convergent validity in terms of high correlation between the LARS score and quality of life (perfect fit 55.5% vs. moderate fit 37.6% vs. no fit 6.8%, respectively; P < 0.001). The LARS score also showed good discriminative validity between groups of patients differing by sex (29 for males vs. 25 for females; P = 0.014), tumor level (29 for ≤8 cm vs. 24 for >8 cm; P = 0.021), and radiotherapy (32 for yes vs. 24 for no; P = 0.001). The LARS score also demonstrated high reliability at test-retest with no difference between scores at the first and second tests (intraclass correlation coefficient: Q1 = 0.932; Q2 = 0.909, Q3 = 0.944, Q4 = 0.931, and Q5 = 0.942; P < 0.001, respectively).
Conclusion
The Korean version of the LARS score questionnaire has proven to be a valid and reliable tool for measuring LARS in Korean patients with rectal cancer.
INTRODUCTION
Treatment of colorectal cancer has gradually improved worldwide over the past few decades. Korea was the most successful country at increasing the survival of patients with colorectal cancer between 2011 and 2015 [1]. Due to advancements in chemotherapy, radiotherapy, and surgical techniques, more sphincter-saving surgeries using a colorectal or coloanal anastomosis without a permanent stoma have been performed for patients with rectal cancer. However, patients who underwent radical surgery for rectal cancer often suffer from low anterior resection syndrome (LARS) and symptoms such as frequent bowel movements, gas incontinence, fecal incontinence, fragmentation, and urgency [2-5].
Improved quality of life (QoL) and oncologic outcomes are important metrics for survivors of rectal cancer. Many patients who undergo surgery for rectal cancer experience LARS, and a number of studies reported that bowel habit changes affect patient QoL [6-8]. Until Emmertsen et al. [2] developed the LARS score in 2012, there were no evaluation tools or questionnaires for fecal incontinence and QoL that showed feasibility or validity for direct evaluation of LARS. The LARS score accurately reflects severity of bowel dysfunction after rectal surgery by scoring symptoms of LARS. The LARS score questionnaire was developed in Danish and was validated in many languages including English, Swedish, Spanish, Dutch, Japanese, and Chinese [9-12]. However, there has been no validated Korean version of the LARS score questionnaire until now. The aim of this study was to investigate the convergent validity, discriminative validity, and reliability of the Korean version of the LARS score questionnaire.
METHODS
Questionnaire
The LARS score is composed of five items: (1) incontinence for flatus, (2) incontinence for liquid stools, (3) frequency of bowel movements, (4) clustering of stools, and (5) urgency [2]. Each item has 3 or 4 response choices that are assigned with different score values. The range of score values was 0 to 42 with limits of 0 to 20 (no LARS), 21 to 29 (minor LARS), and 30 to 42 (major LARS). We received permission from Emmertsen et al. to translate the English version of the LARS score questionnaire into Korean. The translation was performed according to the forward-and-back translation method and followed the recommendations of the World Health Organization and the European Organisation for Research and Treatment of Cancer (EORTC) [13,14]. Two independent translators whose native language is Korean translated the English version of the LARS score into Korean. After the two translators reached an agreement, a common version was back-translated to English by a third independent translator whose native language is English. Additional explanations for confusing words were inserted in parentheses in the final Korean version because “less than once” could be misunderstood in the Korean language. “Less than once” is usually regarded as “no or zero” in Korean. Appendix 1 shows the final Korean version of the LARS score questionnaire. One anchor question to assess QoL was added to the last part of the questionnaire (“Overall, how much does your bowel function affect your QoL?”), as previous studies suggested [9-11]. Available responses were “No”/“A little”/“Some”/ “A lot.”
Patients
Between January and December 2018, patients who underwent radical surgery for rectal cancer at five institutions in Korea answered the Korean version of the LARS score questionnaire, including an anchor question. Participants were retested once more after 2 weeks. All participants had undergone a curative total mesorectal excision for rectal adenocarcinoma less than 15 cm from the anal verge. Only patients who had no stoma at the time of answering the questionnaire were eligible, including those who previously received stoma takedown (repair). Patients who had undergone abdominoperineal resection or palliative surgery were excluded. Patients who underwent surgery or examinations that could affect bowel function in the time between test and retest were also excluded to prevent test-retest reliability bias.
This study was conducted in compliance with the principles of the Declaration of Helsinki. The study protocol was reviewed and approved by the Institutional Review Boards of participating institutions (IRB No. of the principal investigator: KHNMC 2017-10-009-001). Written informed consents were obtained.
Convergent validity
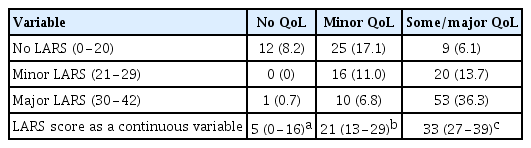
Associations among the three groups based on the LARS score (no, minor, and major) and the three groups from the QoL impact anchor question responses (no, a little = minor, some/a lot = some/major) were analyzed for convergent validity. A 3-by-3 table was created to assess correlation between the LARS groups and the QoL groups. The percentages of perfect fit, moderate fit, and no fit were calculated. We hypothesized that the severity of the LARS score and influence on QoL would match. Therefore, when “no LARS” matched with “no affect to QoL,” a perfect fit was achieved. A mismatch in one or two categorical levels was regarded as moderate fit or no fit, respectively. Moreover, differences in LARS score and QoL as continuous variables were also investigated.
Discriminative validity
Discriminative validity was assessed by comparing groups using the LARS score as a continuous variable: sex, age (more or less than 70 years), presence of stoma, time using the anus (i.e., time since radical surgery without stoma or reversal surgery of temporary stoma, less than or greater than 18 months), tumor location (higher or lower than 8 cm from the anal verge), and radiotherapy.
Test-retest reliability
Agreement between the first and second responses was investigated as perfect, moderate, and no agreement. The same response for both the first and second test was regarded as perfect agreement. A difference of one category or two categories was regarded as moderate or no agreement, respectively. The extent of agreement was demonstrated on a Bland-Altman plot. In addition, the intraclass correlation coefficient (ICC) of each question was assessed.
Statistical analyses
The sample size was calculated according to the rule that at least 10 to 15 patients per question should be recruited for appropriate analysis in the questionnaire survey [15]. The difference in convergent validity was analyzed via linear association and the Spearman correlation coefficient. Additionally, the median value and interquartile range (IQR) were represented as a box plot, and the difference was assessed by Kruskal-Wallis and Mann-Whitney U-tests for convergent and discriminative validity. For test-retest reliability, the extent of agreement was demonstrated on a Bland-Altman plot and the ICC of each question was assessed. All statistical analyses were performed with the IBM SPSS ver. 24.0 (IBM Corp., Armonk, NY, USA) and R software packages (R version 3.5.1, www.r-project.org).
RESULTS
Patients
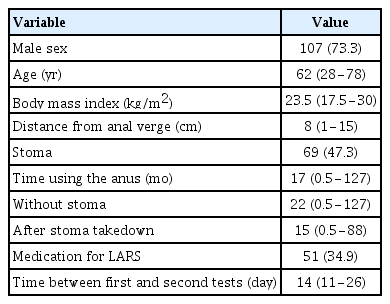
During the study period, 173 patients were asked to answer the Korean version of the LARS score questionnaire. Among them, 146 patients (84.4%) completed the questionnaire twice. Patient and treatment characteristics are listed in Table 1. The median age was 62 years, and the median tumor location was 8 cm from the anal verge. According to the LARS score, 46 patients had no LARS (31.5%), 36 had minor LARS (24.7%), and 64 had major LARS (43.8%).
Convergent validity
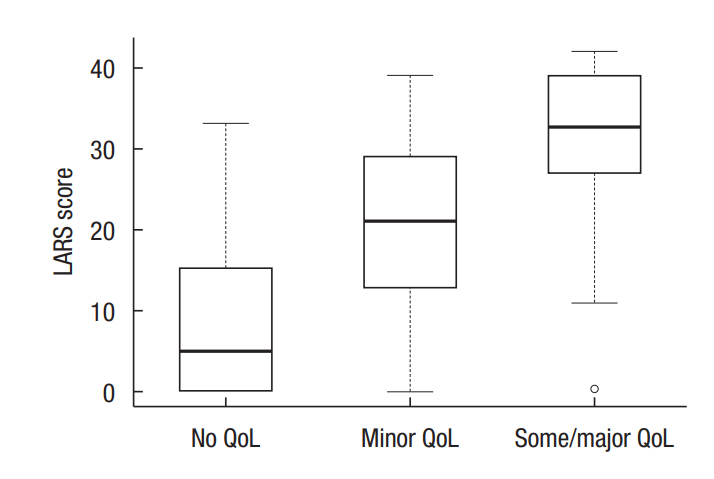
For QoL categorization, perfect, moderate, and no fit were 55.5%, 37.6%, and 6.8%, respectively (P < 0.001) (Table 2). The median LARS scores for no impact on QoL, minor impact on QoL, and some/major impact on QoL significantly differed at 5, 21, and 33, respectively (Fig. 1).
Discriminative validity
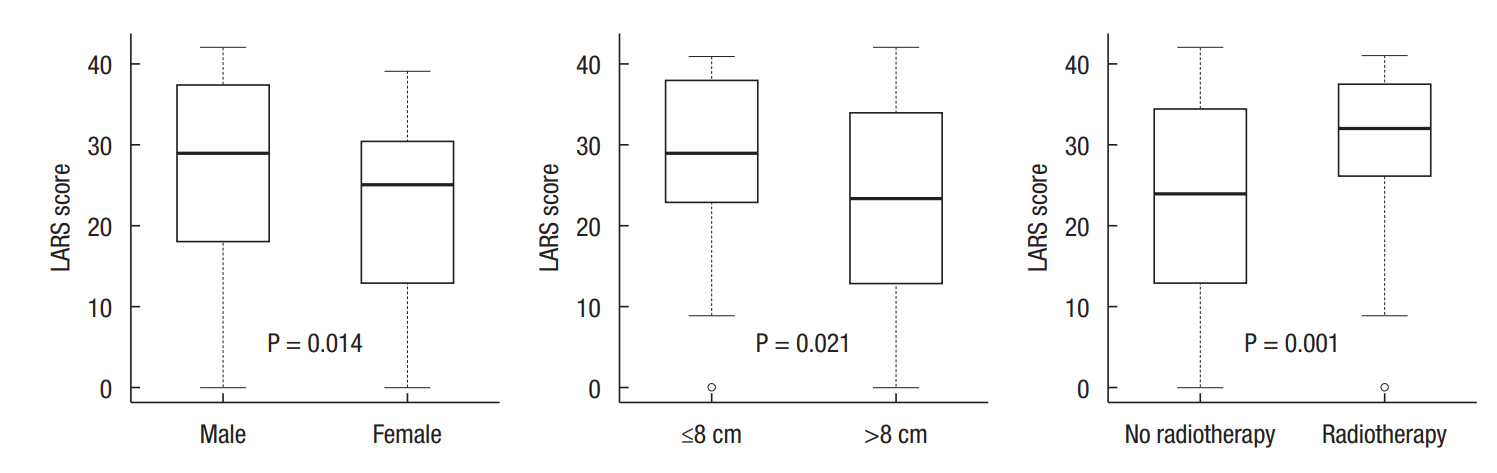
Male sex, tumor location less than 8 cm from the anal verge, and radiotherapy showed a significantly lower LARS score (Fig. 2). The LARS score was not different between patients grouped according to age, presence of stoma, and time using the anus.
Test-retest reliability
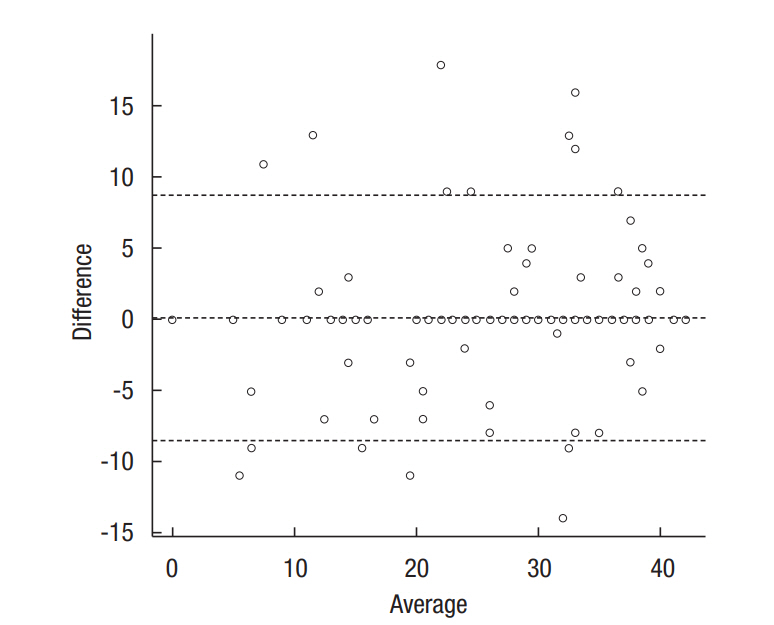
For overall LARS category, perfect fit was 88.3% (Table 3). Each of the five items showed a high proportion of perfect fit (85.6%− 91.1%). The ICCs ranged from 0.909 to 0.944, demonstrating high agreement. Fig. 3 illustrates the extent of agreement between LARS scores on the first and second tests via the Bland-Altman plot.
DISCUSSION
This study validated the Korean version of the LARS score questionnaire in Korean patients with rectal cancer. The LARS score questionnaire translated from English to Korean demonstrated convincing psychometric properties regarding convergent validity, discriminative validity, and reliability, in accordance with previous validation studies.
Perfect, moderate, and no fit were 55.5%, 37.6%, and 6.8%, respectively, between the LARS and QoL groups for convergent validity (Table 3). The LARS score was clearly associated with QoL in Korean patients, and these results are similar to studies from Europe and Japan. Juul et al. [9] reported that there were 4.3% “no fits” in Sweden, 6% “no fits” in Spain, 7.7% “no fits” in Germany, and 2.3% “no fits” in Denmark. However, Akizuki et al. [11] reported only 0.7% “no fits”; “perfect” and “moderate fit” were similar to our results (52.9% and 46.3%, respectively). Previous studies demonstrated that bowel dysfunction after rectal surgery is highly associated with poor QoL [7,8,16]. These studies analyzed the correlation of LARS score with EORTC Quality of Life Questionnaire-Core 30 and/or the Wexner incontinence score. Based on these results, surgeons can actively, rather than passively, help patients improve their QoL.
We sought to assess discriminative validity by identifying the correlation between the LARS score and known variables that might affect bowel dysfunction, including gender, age, presence of stoma, time using the anus, tumor location, and radiotherapy. Male gender, lower tumor location, and radiotherapy were related with lower LARS score in this study. In the European population, age, radiotherapy, and type of surgery (total vs. partial mesorectal excision) showed differences in the LARS score [9,10]. However, we could not analyze the type of surgery as in the European validation study because the concept of partial mesorectal excision was unfamiliar. This was similar to a Japanese validation study which classified the type of surgery as (low) anterior resection, ultra-low anterior resection, and intersphincteric resection because the concept of partial mesorectal excision is relatively new in Japan [11]. Limitations of their study included a study population from a single center and use of few neoadjuvant therapies. Hou et al. [12] demonstrated that female gender, radiotherapy, longer length of postoperative period, and lower tumor level were related to a lower LARS score in Chinese patients. Conversely, patients younger than 66.5 years old reported higher LARS scores than older patients with borderline significance (P=0.051). Although female gender is related to poor bowel dysfunction, our results may be due to confounding factors. There were several differences between male and female patients in our dataset, including age (62 years vs. 60 years), radiotherapy (37.4% vs. 28.2%), and time using the anus (15 months vs. 24 months); male patients tended to have poorer factors, which may be responsible for the higher LARS scores.
In terms of test-retest reliability, we found relatively higher agreement (perfect fit more than 85% for all items) compared with European and Japanese studies. These results could be due to our strict time interval requirements between the first and second tests. Because bowel preparation for colonoscopy or any surgery under general anesthesia can influence the reliability of the test, patients who were scheduled to receive procedures or surgery were excluded. Accuracy and simplicity of translation were also important to achieving reliability; the self-reported questionnaire was easily understood by the non-medical population.
This is the first multicenter study to validate the Korean version of the LARS score questionnaire under permission of the original authors. Physicians, nurses, and students can now freely use this Korean version of the LARS score questionnaire without contacting us. We hope that this questionnaire will assist in educating medical personnel and helping patients who suffer from LARS.
In conclusions, the Korean version of the LARS score questionnaire has proven to be a valid and reliable tool for measuring LARS in Korean patients with rectal cancer. This questionnaire is helpful for colorectal surgeons to improve treatment strategies for patients who suffer from LARS.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government Ministry of Education (No. 2017R1D1A1B03030948).
References
Appendices
Appendix 1.
The Korean version of the low anterior resection syndrome score questionnaire
ac-2019-08-01-app1.pdf