Total neoadjuvant therapy for rectal cancer: evidence and challenge
Article information
Abstract
Recent advances in the management of rectal cancer have dramatically changed the clinical practice of colorectal surgeons because the main focus of rectal cancer treatment has changed from sphincter-saving to an organ-preserving strategies. Modifying the delivery of systemic chemotherapy to improve patients’ survival is another progress in colorectal cancer management, known as total neoadjuvant therapy (TNT). TNT is a new strategy used by colorectal surgeons to improve the quality of life and survival of patients after treatment. TNT poses limitations or obstacles, such as overtreatment issues in patients with stage I rectal cancer. However, considering the quality-of-life issues in patients with low-lying rectal cancer necessitating a permanent colostomy, the indication for TNT will be expanded. This review summarizes the recently conducted clinical trials and foresees future perspectives on TNT.
INTRODUCTION
Advanced rectal cancers were traditionally treated with neoadjuvant chemoradiation therapy (nCRT) followed by total mesorectal excision (TME) and postoperative adjuvant chemotherapy [1, 2]. This multimodal approach significantly improved local control; however, overall survival did not improve, mainly due to distant metastasis after the surgery [3]. Marginal or poor overall survival resulted from micrometastatic disease during the waiting period after the nCRT to surgery and poor compliance with postoperative adjuvant chemotherapy.
Recent evidence has shown a good prognosis when patients achieve pathologic complete response (pCR) after nCRT [4, 5]. The current rectal cancer management should be extended to achieve more pCR and apply the organ-preserving strategy beyond the sphincter-preserving surgery because it harbored many disadvantages, such as low anterior resection syndrome, sexual and voiding difficulties, and permanent colostomy [5, 6].
Various approaches have been tested to improve the survival of rectal cancer patients and have resulted in promising outcomes. A new treatment strategy named total neoadjuvant therapy (TNT), consists of chemotherapy given before or after the CRT to control micrometastatic disease earlier and improve compliance with chemotherapy.
The year 2022 will be a landmark year for the treatment of rectal cancer because the mainstream of rectal cancer management has been changed from nCRT to TNT. The National Comprehensive Cancer Network (NCCN) guideline endorsed the TNT strategy for the circumferential resection margin (CRM) threatening and CRM-negative tumors in the 2022 guideline [7]. These dramatic changes reflected the results of 2 prospective randomized clinical trials, i.e., the RAPIDO (Rectal Cancer and Preoperative Induction Therapy Followed by Dedicated Operation) trial [8] and PRODIGE (Partenariat de Recherche en Oncologie Digestive) 23 trial [9] in 2021. The CAO/ARO/AIO-12 trial [10, 11] and OPRA (Organ Preservation of the Rectal Adenocarcinoma) trial [12] comparing the induction and consolidation chemotherapy as a TNT have been reported. This review summarizes the recently reported clinical trials and foresees the future perspectives on TNT.
TNT FOLLOWED BY TME VERSUS NCRT FOLLOWED BY TME AND POSTOPERATIVE CHEMOTHERAPY
RAPIDO trial (2021)
The RAPIDO trial [8] compared the short-course radiation therapy followed by consolidation chemotherapy with standard long-course nCRT. The standard nCRT group received long-course CRT (either 50.4 Gy with 28 fractions or 50.0 Gy with 20 fractions) with capecitabine (twice daily oral capecitabine 825 mg/m2 during the radiation period) followed by TME within 6 to 10 weeks after CRT was completed. Postoperative adjuvant chemotherapy is either 8 cycles of CAPOX (capecitabine and oxaliplatin; capecitabine 1,000 mg/m2 orally twice daily on days 1–14 and oxaliplatin 130 mg/m2 intravenously on day 1) or 12 cycles of FOLFOX4 (fluorouracil, leucovorin, and oxaliplatin; oxaliplatin 85 mg/m2 intravenously on day 1, leucovorin 200 mg/m2 intravenously on days 1 and 2, followed by bolus fluorouracil 400 mg/m2 intravenously and fluorouracil 600 mg/m2 intravenously for 22 hours on days 1 and 2) administered within 6 to 8 weeks after surgery. The experimental group received short-course radiation therapy followed by either 6 cycles of CAPOX or 9 cycles of FOLFOX4. Surgery was performed after 2 to 4 weeks after the completion of chemotherapy. The primary endpoint was a 3-year disease-related treatment failure defined as the first occurrence of locoregional failure, distant metastasis, a new primary colorectal tumor, or treatment-related death.
Among the 920 enrolled patients, 912 were eligible for the analysis (462 in the experimental group and 450 in the standard group). The 3-year disease-related treatment failure rate was 23.7% in the experimental group, whereas 30.4% in the standard group, showing a statistically significant difference (P=0.019) with a median follow-up of 4.6 years. This promising result supported TNT can be considered a new standard treatment of rectal cancer, especially for high-risk locally advanced rectal cancers defined as cT4 or N2 disease, CRM-threatening, extramural vascular invasion–positive, and lateral pelvic node enlargement, and ugly rectal cancers.
PRODIGE 23 trial (2021)
The PRODIGE 23 trial [9] compared the triplet induction chemotherapy followed by long-course CRT and TME with standard nCRT followed by TME. The standard nCRT group received long-course CRT with capecitabine (twice daily oral capecitabine 825 mg/m2 during the radiation period) followed by mandatory TME within 6 to 8 weeks after CRT was completed postoperative adjuvant chemotherapy is either 8 cycles of capecitabine (capecitabine 1,250 mg/m2 orally twice daily on days 1–14) or 12 cycles of FOLFOX6 (oxaliplatin 85 mg/m2 intravenously on day 1, leucovorin 400 mg/m2 intravenously on days 1 and 2, followed by bolus fluorouracil 400 mg/m2 intravenously and fluorouracil 2,400 mg/m2 intravenously for 46 hours) administered within 5 to 12 weeks after surgery. The experimental group received the triplet induction chemotherapy with FOLFIRINOX (oxaliplatin 85 mg/m2 intravenously, irinotecan 180 mg/m2, leucovorin 400 mg/m2, and fluorouracil 2,400 mg/m2 intravenously every 14 days for 6 cycles) followed by CRT (50 Gy for 5 weeks and 800 mg/m2 concurrent oral capecitabine twice daily for 5 days per week), TME, and adjuvant chemotherapy either 6 cycles of modified FOLFOX6 or oral capecitabine. The primary endpoint was 3-year disease-free survival (DFS).
A total of 461 patients were randomly assigned to either the experimental induction chemotherapy group (n=231) or standard group (n=230). The 3-year DFS rate was 75.5% in the experimental induction chemotherapy group and 68.5% in the standard nCRT group, showing a statistically significant difference (P=0.034), with a median follow-up of 46.5 months. Although the triplet induction chemotherapy group had slightly more high serious adverse events than the standard group (27% vs. 22%, respectively), these differences were not statistically significant.
CONSOLIDATION OR INDUCTION CHEMOTHERAPY
To date, 2 randomized clinical trials have head-to-head compared induction and consolidation chemotherapy as a TNT.
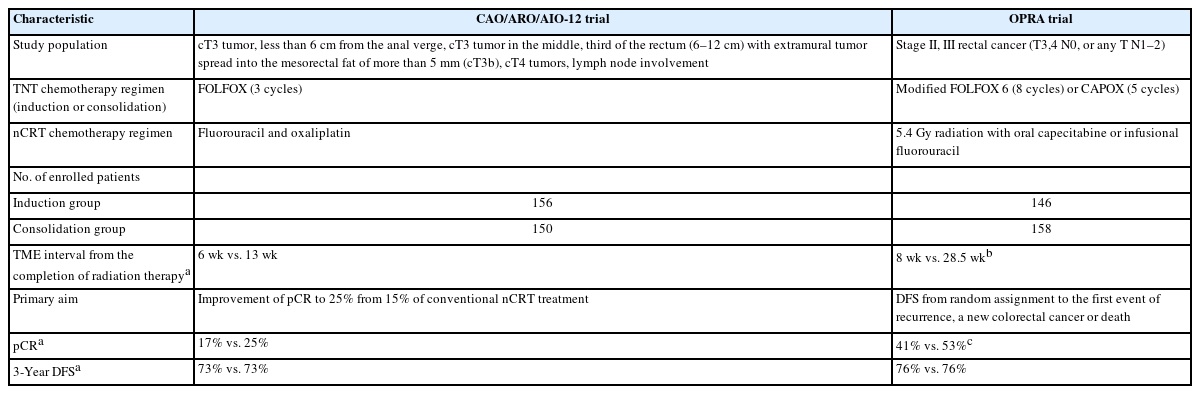
CAO/ARO/AIO-12 trial (2019, 2022)
The CAO/ARO/AIO-12 German trial [10, 11] compared 3 cycles of induction chemotherapy with FOLFOX (oxaliplatin 100 mg/m2, leucovorin 400 mg/m2 intravenously followed by fluorouracil 2,400 mg/m2 intravenously for 46 hours) followed by nCRT adding fluorouracil and oxaliplatin (continuous infusion of 250 mg/m2 of fluorouracil on days 1–14 and days 25–35 of radiotherapy and 2-hour infusion of 50 mg/m2 oxaliplatin on day 1, 8, 22, and 29 of radiotherapy) with nCRT adding fluorouracil and oxaliplatin and 3 cycles of consolidation chemotherapy with same FOLFOX. Mandatory TME was scheduled approximately at the 18th week after TNT initiation in both groups. The primary aim of the trial was to identify the most promising TNT sequence which can increase pCR to 25% from 15% after conventional nCRT using a pick-the-winner design.
The pCR rates achieved in 17% and 25% of the induction and consolidation chemotherapy groups, respectively. When clinical complete response (cCR) was included in both groups, combined complete response was increased to 21% for the induction and 28% for the consolidation group. However, the 3-year DFS rate was 73% in both groups after a median follow-up of 43 months [11].
OPRA trial (2022)
The OPRA trial [12] was also a head-to-head comparison of induction and consolidation chemotherapy with long-course radiation therapy and either TME or watch-and-wait on the basis of tumor response and aimed at comparing the 3-year DFS and TME-free survival. Both groups received the same 8 cycles of FOLFOX or 5 cycles of CAPOX chemotherapy as a TNT strategy. Patients received radiation therapy of up to 50.6 Gy with either infusional fluorouracil or oral capecitabine. The primary endpoint of the OPRA trial was 3-year DFS. Unlikely the other studies, after the completion of TNT, the investigators restaged the patients for approximately 8 weeks to confirm the cCR for organ preservation purposes. Once the patients achieved cCR or near cCR, they followed a watch-and-wait strategy.
The 3-year DFS rate was identical in both groups (76%). The TME-free survival was significantly better in the consolidation group (53%) than in the induction group (41%).
DISCUSSION
The RAPIDO trial is promising for supporting short-course radiation therapy followed by consolidation chemotherapy regarding the 3-year disease-related treatment failure. The RAPIDO trial was similar to the Polish II trial [13], which supported short-course radiation therapy and consolidation chemotherapy. The Polish II trial also reported a better 3-year overall survival than the standard long-course nCRT treatment group. The Polish group suggested the role of radiation in activating the antitumor immune response during the waiting period before surgery. The role of radiation therapy in rectal cancer treatment is challenging now, mainly because of the radiation-related toxicities (PROSPECT trial, ClinicalTrials.gov identifier: NCT01515787) [14]. The ongoing trial would define the role of radiation therapy.
The role of the immune response has already been proven in a recent immunotherapy trial in patients with mismatch-deficient rectal cancer [15]. The PRODIGE 23 trial introduced very aggressive triplet chemotherapy of oxaliplatin, irinotecan, and fluorouracil. Only 13% of the patients in the study population were older than 70 years, therefore, serious adverse events were similar to those in the nCRT group. The actual study populations differed between the RAPIDO and the PRODIGE 23 trials; however, both trials included an advanced rectal cancer population with the CRM-threatening rectal cancers. The authors of both trials suggested the use of TNT for advanced rectal cancer based on their excellent results.
The RAPIDO trial also included an old age population (defined as aged more than 65 years) of approximately 40%, and the median age of both trials was 62 and 61 years, respectively. This suggested that TNT would benefit younger patients with advanced rectal cancer who can tolerate prolonged chemotherapy. The role of TNT in the older age population is not well established despite excellent and well-conducted clinical trials (Table 1).
The sequence of chemotherapy has long been questioned among TNT supporters. The 2 trials (CAO/ARO/AI-12 and OPRA) showed nearly identical results of 3-year DFS rates of 73% to 76%. From the perspective of organ preservation, both trials suggested that consolidation chemotherapy was better than induction chemotherapy because the waiting period for surgery after the completion of radiation therapy was longer in the consolidation group suggesting the role of radiation therapy in cCR achievement (Table 2).
However, recent trials have had some drawbacks, particularly regarding complicated chemotherapy regimens among studies. Most trials have adopted the FOLFOX regimens. The PRODIGE 23 trial adopted triplet chemotherapy (FOLFIRINOX), whereas the German trial adopted fluorouracil and oxaliplatin–based nCRT. Many previous trials already have reported higher chemotherapy-related toxicities of oxaliplatin during the long-course nCRT [16–19], while the German trial insisted on the administration of oxaliplatin during the nCRT. The advantages and disadvantages of adding oxaliplatin during the nCRT should be clarified in future studies. Moreover, the aims of the trials differed, so aims should be decided based on the patients’ perspectives.
There are concerns regarding overtreatment with the TNT strategy. However, most trials included patients with high-risk advanced low rectal cancer. The overtreatment issue will continue, but it will not compromise the role of TNT in organ preservation or long-term survival in the future. As the older patients with advanced rectal cancer increased during the days, the optimal treatment for old-age advanced rectal cancer patients remained unsolved and should also be addressed in future trials.
However, the recent trials did not provide better survival of TNT-treated patients compared to the historical control or the standard treatment groups, and the data did not mature fully until we reached more solid conclusions. This problem will be addressed in the future studies.
CONCLUSION
TNT has many theoretical advantages, such as improved chemotherapy compliance, early control of micrometastatic diseases, and higher pCR or cCR rates in aggressive low rectal cancer patients. The upcoming results from ongoing trials will provide more evidence regarding the advantages and disadvantages of TNT. Moreover, the indication for TNT for low rectal cancer will expand beyond sphincter-saving surgery for rectal cancer to achieve organ preservation of the rectum.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
This work was partly funded by the KONCLUDE (KCSP Trial of Consolidation Chemotherapy for Locally Advanced Mid or Low Rectal Cancer after Neoadjuvant Concurrent Chemoradiotherapy) trial research fund from the Korean Society of Coloproctology.