Optimal anastomotic technique in rectal surgery to prevent anastomotic leakage
Article information
Abstract
Complications after colorectal surgery remain inevitable, and anastomotic leakage is one of the most severe and potentially fatal complications. Generally, anastomotic leakage is associated with severe peritonitis, the need for emergency reoperation, and an increased mortality rate. Additionally, particularly after rectal cancer surgery, it has a negative impact on long-term outcomes, including postoperative anorectal function, local recurrence, and survival. To prevent anastomotic leakage, understanding the characteristics of each anastomotic technique and establishing a stable anastomotic procedure are important. Transanal total mesorectal excision (TaTME) is a relatively new advanced surgical access technique for pelvic dissection and facilitates different anastomotic techniques without the need for transabdominal rectal transection. Especially, stapled anastomosis in TaTME, also known as double purse-string circular stapled anastomosis or the single stapling technique (SST), has gained much attention as an alternative to the conventional double stapling technique (DST). In this article, we describe the DST, SST, and hand-sewn anastomosis as anastomotic techniques after rectal surgery, focusing mainly on the differences between conventional anastomotic techniques and SST in TaTME. Furthermore, the blood flow evaluation method for the reconstructive colon before anastomosis, which is extremely important in anastomotic leakage prevention regardless of the anastomotic type, is also described.
INTRODUCTION
Complications after colorectal surgery are still inevitable, and anastomotic leakage (AL) is one of the most severe and potentially fatal complications. The reported incidence rate is approximately 2.8% to 30% in total, 75% of which occurs in rectal anastomosis, resulting in a mortality rate of 2% to 16.4% and a morbidity rate of 20% to 35% [1–5]. Generally, AL is associated with severe peritonitis, emergency reoperation, and an increased mortality rate. Additionally, particularly after rectal cancer surgery, AL has a negative impact on both short and long-term outcomes including postoperative anorectal function [6], local recurrence, and survival [7]. To prevent AL, it is important to understand the characteristics of each anastomotic technique and to establish a stable anastomotic procedure.
Transanal total mesorectal excision (TaTME) is a relatively new advanced surgical access technique for pelvic dissection and facilitates different anastomotic techniques without the need for transabdominal rectal transection [8, 9]. TaTME is a promising approach pioneered to tackle the challenges posed by difficult pelvic dissections in rectal cancer and the restrictions in angulation of currently available laparoscopic staplers, particularly in a narrow pelvis [10]. The standard TaTME technique incorporates an open rectal stump with continuity restored by a coloanal hand-sewn or colorectal stapled anastomosis. Specifically, stapled anastomosis in TaTME is called double purse-string circular stapled anastomosis or the single stapling technique (SST) [11].
In this article, we describe the double stapling technique (DST), SST, and hand-sewn anastomosis as anastomotic techniques after rectal surgery, while focusing mainly on the differences between conventional anastomotic techniques and SST in TaTME. Furthermore, the blood flow evaluation method for the reconstructive colon before anastomosis, which is extremely important in AL prevention regardless of the anastomotic type, is also described.
DOUBLE STAPLING TECHNIQUE
History of double stapling technique
In 1980, Knight and Griffen [12] reported a colorectal anastomotic technique, in which the anal side of a rectal tumor was transected with a linear stapler, and the staple line was punched out with a circular stapler. Subsequently, in 1983, Cohen et al. [13] named this anastomotic technique as DST. The establishment of DST has enabled safe, reliable, and rapid anastomosis for lower rectal cancer, thus, presenting great benefits to many patients with rectal cancer. It is no exaggeration to state that the circular stapler is one of the greatest medical device innovations of the 20th century in the surgical field.
Surgical procedures of double stapling technique
When using DST for patients with lower rectal cancer, it is important to dissect the perirectal area thoroughly toward the anal side and obtain sufficient mobilization of the rectum to facilitate the subsequent rectal transection. When performing total mesorectal excision (TME), perirectal dissection proceeds toward the anal side up to the endopelvic fascia, which covers the levator ani muscle, at the bilateral anterolateral and posterior sides. The relatively firm smooth muscular tissue that remains on the posterior wall is the rectococcygeal muscle or hiatal ligament (Fig. 1). Resection of this tissue before rectal transection provides additional mobilization of the rectum.
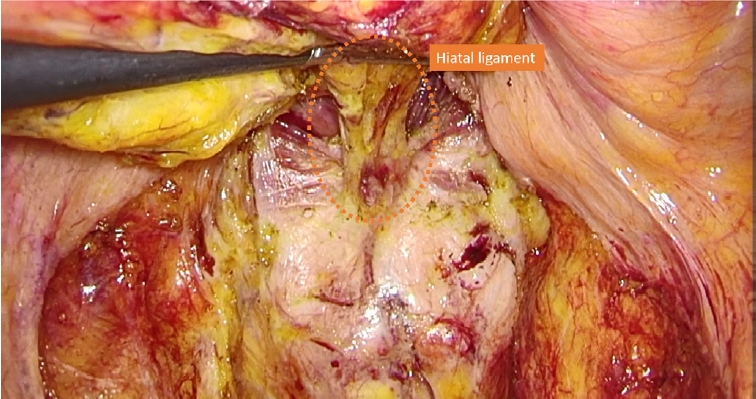
Fig. 1. Representative image of the rectococcygeal muscle or hiatal ligament (orange dotted circle).
When tumor-specific mesorectal excision is performed, the location of the lower edge of the tumor is confirmed by intraoperative colonoscopy or digital rectal examination, and the location of the rectal transection line is decided with a proper distal margin according to each case. The mesorectum should be transected perpendicular to the rectal axis to avoid rectal wall injury, especially thermal injuries caused by contact of the blade of the ultrasonically activated device with the rectal wall.
A detachable intestinal clamper is attached to the anal side of the tumor, and the rectal lumen is irrigated transanally to prevent the implantation of free cancer cells. Then, the rectum is transected with a linear stapler. The circular stapler used for anastomosis is selected according to the diameter of the reconstructive colon.
Risk factors of anastomotic leakage in double stapling technique
Multiple stapler firing
Several studies have shown that multiple stapler firing, i.e., multiple uses of the cartridge of a linear stapler for rectal transection, is a risk factor for AL [14–16]. A recent systematic review summarizing these studies showed that the incidence rates of AL in double stapler firing and single stapler firing were 6.7% and 3.5%, respectively. Double stapler firing was therefore a significant risk factor for AL (odds ratio, 2.44). It was concluded that colorectal surgeons should strive to reduce the number of cartridges used and transect the rectum with single stapler firing during rectal surgery [15]. This discussion is based on the inferior strength of the intersection of staple lines when compared to that with single stapler use. Conversely, Kuroyanagi et al. [17] introduced the technique of “planned double stapled firing,” which intentionally uses 2 cartridges for rectal transection, followed by punching out the intersection of staple lines with a circular stapler. In planned double stapler firing, the use of a 45-mm long cartridge that can be easily maneuvered in the narrow pelvis should be considered. Ito et al. [16] also showed that the use of 3 or more cartridges was an independent risk factor for AL. In addition, in TME, vertical rectal division via the suprapubic port reduced the incidence of multiple stapler firing by 1/3 compared to horizontal rectal division via the right lower abdominal port [16].
In obese male patients with low and bulky tumors and narrow pelvises, it is sometimes difficult to insert the cartridge via the right lower abdominal port perpendicular to the rectal axis, and impossible to transect the rectum with a single stapler firing. To avoid multiple stapler firing, which is a risk factor of AL, the characteristics of each case should be considered to decide on the most appropriate technique to use, such as planned double stapler firing with a 45-mm long cartridge and vertical rectal division via the suprapubic port.
Dog ear
In DST, a so-called “dog ear” is formed at both edges of the rectal stump that cannot be punched out with a circular stapler. Dog ears are considered a risk factor for AL in DST, and several efforts have been made to prevent AL related to the dog ear. One such effort is the use of a linear stapler with the reinforcement cartridge pre-attached to bioabsorbable polyglycolic acid felt when the rectum is transected. The reinforcement cartridge has previously been shown to be effective in preventing air leakage from lung resection margins in respiratory surgery [18] and dehiscence of staple lines in sleeve gastrectomy in bariatric surgery [19]. Yamamoto et al. [20] reported a lower incidence rate of AL (1 of 62 cases [1.6%]) with the use of the reinforcement cartridge for rectal transection in rectal surgery. A prospective multicenter observational study to evaluate the efficacy and safety of the reinforcement cartridge for AL (trial ID: UMIN000029543) is also underway in Japan, and the results of this study are awaited.
The lateral invagination technique (LIT) is another effort to prevent dog ear-related AL. In the LIT procedure, the bilateral dog ears are invaginated centrally using single suturing. Then, both staple line and dog ears are punched out with a circular stapler (Fig. 2). Several case series and retrospective studies have shown the safety and clinical benefit of LIT [21–23]. The ILAC trial, an international multicenter randomized controlled trial (RCT) comparing the conventional DST to DST with LIT for colorectal surgery, is currently underway at Hospital Clinic de Barcelona in Spain to evaluate the advantage of the DST with LIT over the conventional DST. The incidence of AL is the primary outcome of the study. The results of the ILAC trial are awaited.
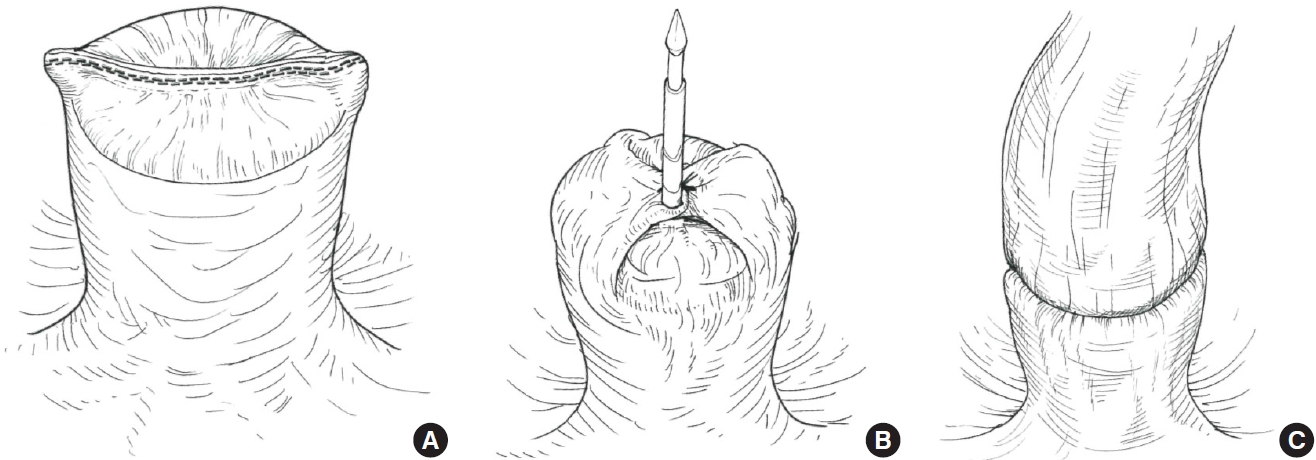
Schema of the lateral invagination technique. (A) Complete the rectal transection as usual double stapling technique. (B) After the bilateral dog ears are invaginated centrally with single suturing, the rectal stump was penetrated with the center shaft of the circular stapler. (C) Both staple line and dog ears are punched out with a circular stapler.
SINGLE STAPLING TECHNIQUE
End-to-end vs. side-to-end
The intersection of the linear staple line and circular staple line in DST is considered a similar risk factor for AL to that of the intersection of linear staple lines in multiple stapler firing described above. Side-to-end stapled anastomosis allows avoidance of the formation of this intersection. Numerous RCTs have compared end-to-end with side-to-end anastomosis in rectal surgery, and a meta-analysis of these trials concluded that side-to-end anastomosis significantly reduced AL after rectal surgery compared to end-to-end anastomosis [24]. However, it is assumed that a certain number of patients in the end-to-end anastomosis group had insufficient blood flow at the end of the reconstructive colon used for anastomosis, and this was considered a confounding factor. Careful interpretation is required to determine the contribution of avoiding the intersection of the staple line to the results.
Single stapling technique in transanal total mesorectal excision
With technological innovations in rectal surgery, surgical approaches have shifted from open to laparoscopic to robotic surgery; however, the biggest breakthrough in rectal cancer surgery in recent years is TaTME, as reported by Sylla et al. [8] in 2010. TaTME is a relatively new surgical approach developed to overcome the technical difficulties of conventional surgical approaches in highly challenging cases. These cases include obese male patients with narrow pelvises and very low and bulky tumors. This is performed by approaching the rectum and mesorectum transanally via an endoscope inserted into the anus. The excellent visibility and maneuverability in the deep pelvis achieved by TaTME are expected to provide oncological advantages in securing sufficient margins from the tumor and functional advantages in preserving autonomic nerves. Penna et al. [11] introduced 4 different anastomotic techniques for TaTME in 2016. Among them was the stapled anastomosis combining a transanal purse-string closure for the rectal cuff and circular stapler firing, which is called double purse-string circular stapled anastomosis or SST. This technique is expected to reduce anastomosis-related complications and increase safety during rectal cancer surgery by overcoming the aforementioned limitations of conventional DST. SST is classified into the following 2 methods according to the direction of connection between the anvil and center shaft of the circular stapler: abdominal double purse-string circular stapled anastomosis, in which the anvil and center shaft are connected from the abdominal side; and transanal pull-through circular stapled anastomosis, in which the anvil and center shaft are connected from the perineal side.
Abdominal double purse-string circular stapled anastomosis
The first choice of SST in TaTME is abdominal double purse-string circular stapled anastomosis. Before the anastomotic procedure, a purse-string suture is circumferentially performed on the rectal cuff with a 2-0 monofilament nonabsorbable suture (Fig. 3A). A catheter is inserted transanally into the pelvis and, then, the rectal cuff is manually ligated and closed. Then, the center shaft of the circular stapler is connected to the catheter (Fig. 3B), and the abdominal team retracts the catheter and guides the center shaft into the pelvis (Fig. 3C). Next, the anvil is connected to the center shaft from the abdominal side (Fig. 3D), and the circular stapler is fired. Finally, hand-sewn reinforcement sutures are added with 16 knotted sutures circumferentially to strengthen anastomosis.
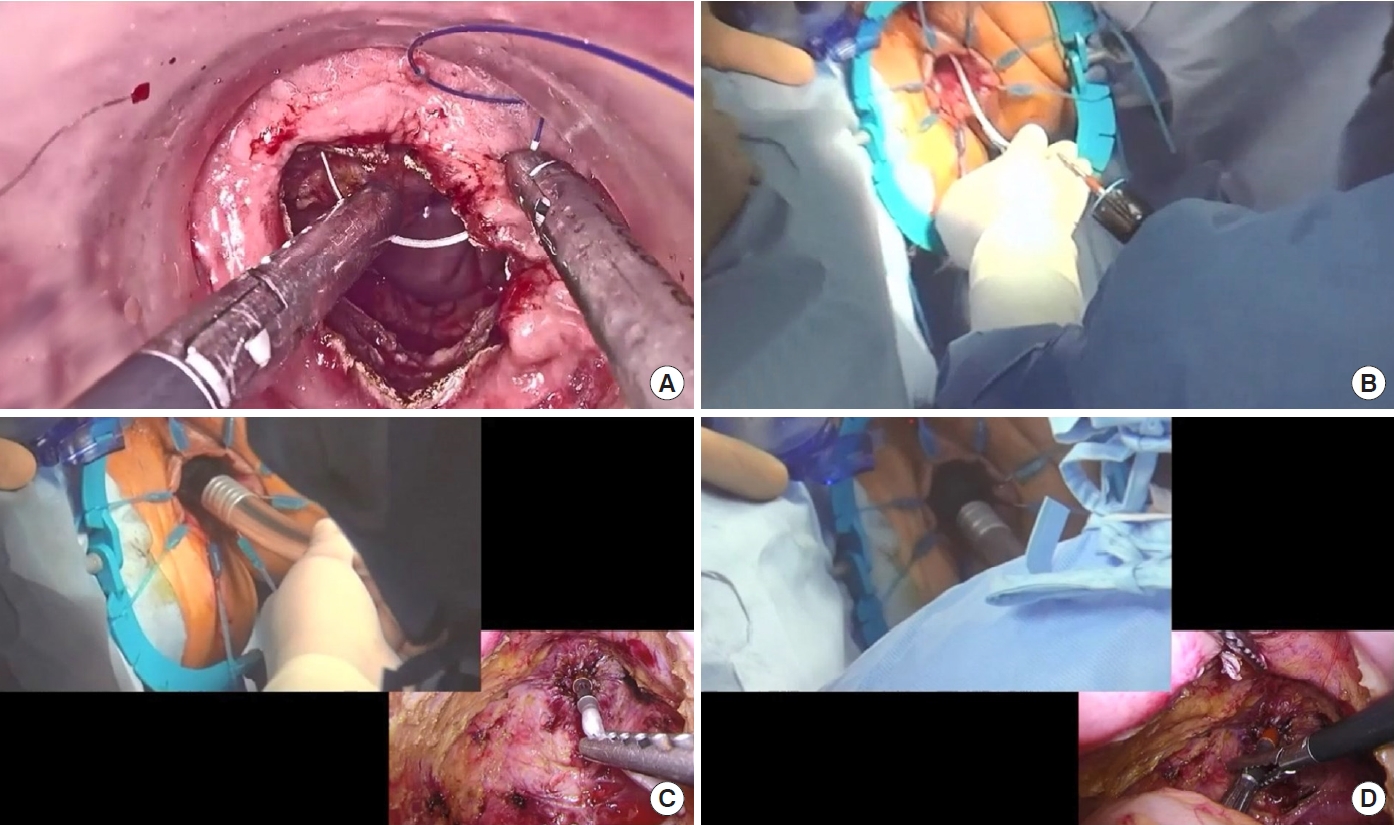
Procedure of abdominal double purse-string circular stapled anastomosis. (A) Before the anastomotic procedure, a purse-string suture is circumferentially performed on the rectal cuff with a 2-0 monofilament nonabsorbable suture. (B) A catheter is inserted transanally into the pelvis, and the rectal cuff is then manually ligated and closed. The center shaft of the circular stapler is connected to the catheter. (C) The abdominal team retracts the catheter and guides the center shaft into the pelvis. (D) The anvil is connected to the center shaft from the abdominal side, and the circular stapler is fired.
Transanal pull-through circular stapled anastomosis
We sometimes encounter situations, in which the anvil cannot be connected to the center shaft of the circular stapler from the abdominal side, because the surgical view in the deep pelvis cannot be obtained from this side. This is especially observed in very low anastomoses in obese male patients with narrow pelvises. In such situations, a transanal pull-through circular stapled anastomosis is considered. A purse-string suture is performed on the rectal cuff with a 2-0 monofilament nonabsorbable suture (Fig. 4A). Before manual ligation, the anvil is pulled out from the perineal side (Fig. 4B) and connected to the center shaft of the circular stapler (Fig. 4C). After connection, the suture is ligated manually (Fig. 4D), and the circular stapler is fired. Finally, hand-sewn reinforcement sutures are added with 16 knotted sutures circumferentially to strengthen the anastomosis, as observed with abdominal double purse-string circular stapled anastomosis.
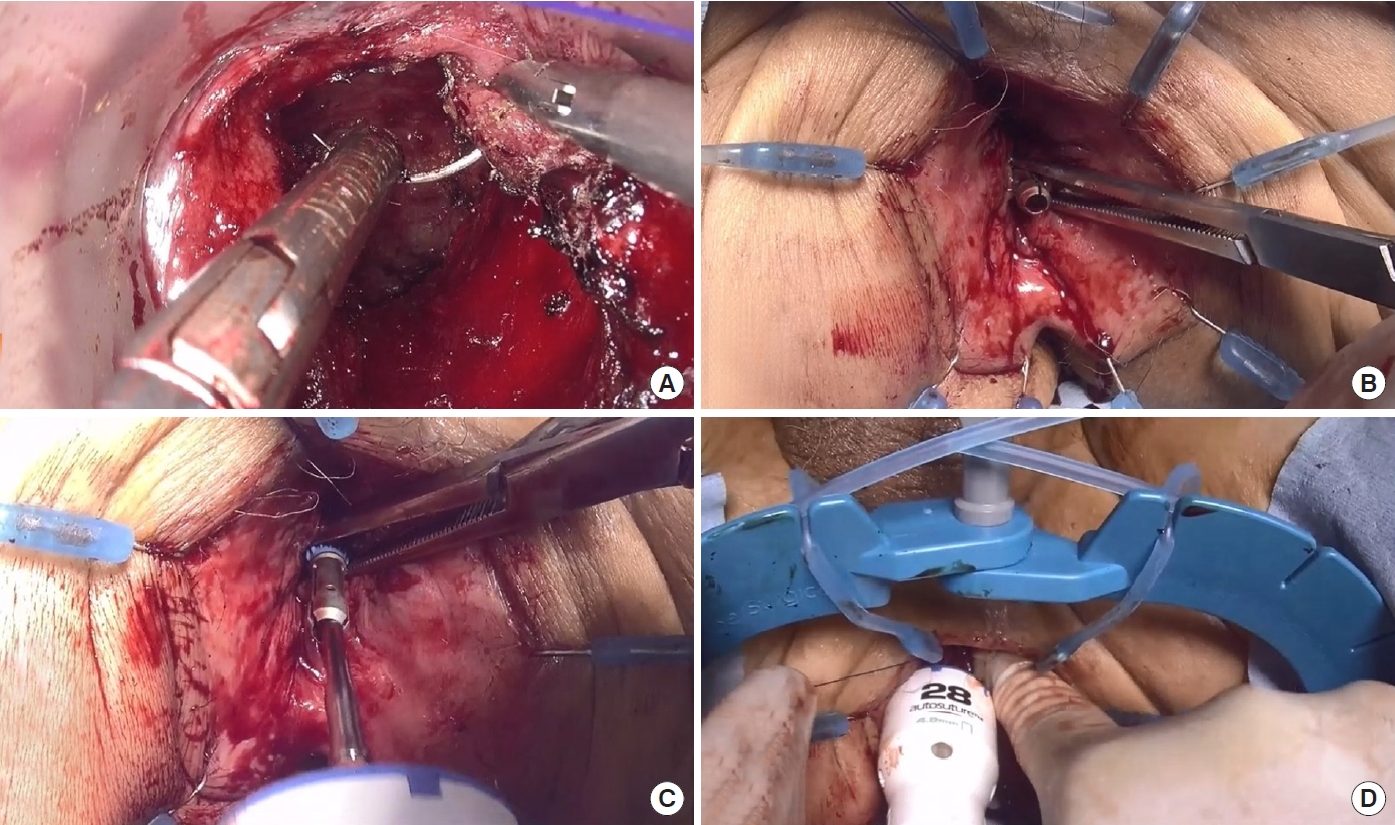
Procedure of transanal pull-through circular stapled anastomosis. (A) A purse-string suture is circumferentially performed on the rectal cuff with a 2-0 monofilament nonabsorbable suture similar to the procedure of abdominal double purse-string circular stapled anastomosis. (B) Before manual ligation, the anvil is pulled out from the perineal side. (C) The anvil, which is pulled out from the perineal side, is connected to the center shaft of the circular stapler. (D) After connection, the suture is ligated manually and the rectal cuff is closed, and finally, the circular stapler is fired.
Single stapling technique vs. double stapling technique in conventional total mesorectal excision
SST in TaTME is an innovative anastomotic technique without the formation of dog ear and staple line intersection. Multiple stapler firing is not required even in difficult cases. Moreover, while DST in conventional TME often requires additional dissection into the anal canal before rectal transection to obtain sufficient mobilization of the rectum, SST in TaTME does not require additional dissection. This may contribute to a reduction in AL and maintenance of postoperative anorectal function because of no deterioration of blood flow to the rectal stump.
A large RCT comparing the outcomes of TaTME to those of conventional laparoscopic TME (LapTME) is currently underway. The COLOR III trial, led by the Netherlands, is an international multicenter RCT that was conducted to evaluate the superiority of TaTME over LapTME for mid and lower rectal cancer, with the primary outcome being local recurrence rate at 3 years of follow-up [25]. The GRECCAR 11 trial in France was a multicenter RCT that was undertaken to evaluate the non-inferiority of TaTME to LapTME, with the primary outcome being the R0 resection rate [26]. Secondary outcomes in both trials include the incidence of postoperative complications and postoperative urinary and anorectal function. It would be beneficial to observe whether TaTME (or SST in TaTME) shows superiority over LapTME (or DST in LapTME), especially in terms of preventing AL.
Super single stapling technique
In the original article introducing 4 anastomotic techniques in TaTME, the abdominal double purse-string circular stapled anastomosis was recommended for patients whose tumor distance from the anorectal junction (ARJ) was 3 to 4 cm. The transanal pull-through circular stapled anastomosis was recommended for a tumor distance of 2 to 3 cm, and hand-sewn anastomosis was recommended for coloanal anastomosis [11]. However, with the technical establishment of the SST in TaTME, the SST can now be performed even in patients whose tumor distance from the ARJ was ≤ 2 cm and in whom hand-sewn anastomosis was originally recommended. We defined SST performed in such patients as “super SST.” According to our definition, the anastomotic line of super SST is located near or below the ARJ. Currently, the Super SST trial (trial ID: UMIN000047818), a multicenter RCT comparing the effects of the super SST versus hand-sewn anastomosis on AL in intersphincteric resection (ISR) with TaTME is underway. As the primary outcome of this trial is anastomosis-related complications within 30 days postoperatively, the results of this trial are awaited to confirm whether super SST is useful in the prevention of AL.
HAND-SEWN ANASTOMOSIS
Indication for hand-sewn anastomosis
Although super SST can be performed even in ISR cases, in total ISR cases, or when the remaining mucosa of the rectal cuff is too fragile to apply a purse-string suture, or when the rectal cuff cannot be ligated and closed owing to high tension, a hand-sewn anastomosis is selected.
Surgical procedures of hand-sewn anastomosis
In our institute, a 3-0 monofilament absorbable suture is used for the anastomosis. First, 8 knotted sutures are applied circumferentially, and the sutures are grasped with mosquito forceps to spread the anastomotic site widely and evenly. Next, 1 or 2 knotted sutures are applied between each of the circumferential sutures, and a hand-sewn anastomosis is completed with a total of 16 or 24 knotted sutures. The sutures for the reconstructive colonic end are applied in all layers, and the sutures for the anorectal end are shallowly applied after the application of the sutures to the levator ani muscle on the cranial side. By suturing the levator ani muscle on the cranial side, the ligation points are moved to the cranial side and the anastomosis is retracted toward the oral side. Contrastingly, if the suture bites for the anorectal end are too large and deep, it will be difficult for the anastomosis to be pulled toward the oral side. Therefore, the suture bite for the anorectal end should be as small and shallow as possible. In this way, we hope to prevent mucosal prolapse after ISR.
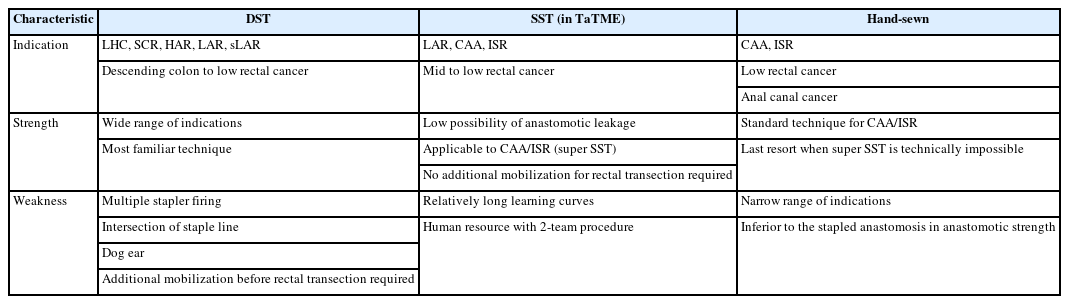
The descriptions of DST, SST (super SST), and hand-sewn anastomosis are summarized. A detailed comparison of the characteristics among DST vs. SST (super SST) vs. hand-sewn anastomosis is presented in Table 1.
BLOOD FLOW EVALUATION BEFORE ANASTOMOSIS
It should be mentioned that proper anastomotic technique is important to prevent AL; however, blood flow is essential to obtain wound healing at anastomosis. AL is inevitable in anastomosis using the ischemic region of the intestinal tract, even if the anastomotic technique is appropriate. Especially, to prevent AL, it is also extremely important to evaluate the blood flow of the reconstructive colon properly.
Indocyanine green fluorescence angiography
Indocyanine green (ICG) bound to plasma proteins has the property of emitting longer wavelength fluorescence (800–850 nm) when excited by near-infrared light (750–810 nm). ICG fluorescence angiography (ICG-FA) is a method used to evaluate tissue blood flow using this property. After intravenous injection of ICG, the presence of blood flow in the reconstructive colon can be observed with a near-infrared scope (Fig. 5).
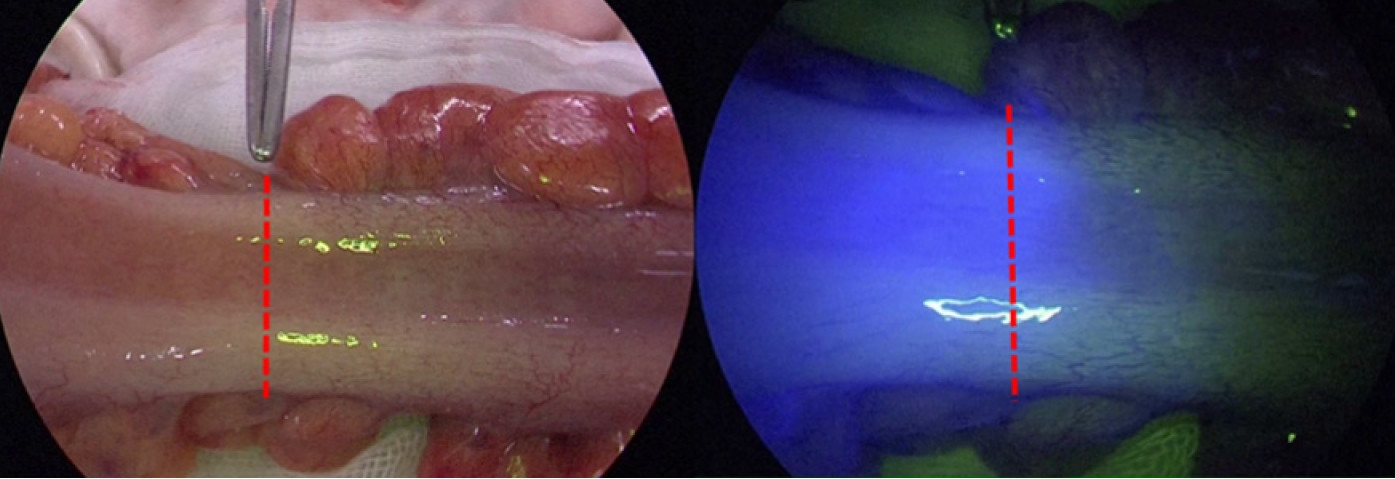
Blood flow evaluation for a reconstructive colon with indocyanine green fluorescence angiography. The region with blood flow emits fluorescence when observed with a near-infrared scope, and the boundary between with and without emission can be observed (red dotted line).
Several studies have reported the usefulness of ICG-FA before anastomosis in preventing AL [27–29]. Additionally, according to our institutional data, a propensity score matching analysis of patients who underwent mid and lower rectal cancer surgery between 2007 and 2017 with blood flow evaluation compared to those without blood flow evaluation of the reconstructive colon using ICG-FA before anastomosis showed that the incidence rates of AL were 13.6% and 2.8% in the groups without and with ICG-FA, respectively. The incidence of AL was significantly lower in the group with ICG-FA [30]. In addition, blood flow evaluation with ICG-FA before anastomosis resulted in a change in the initial transection line of the reconstructive colon in 17% of cases [30]. Especially, it is inadequate to rely on the surgeons’ naked eye to evaluate blood flow. We concluded that intraoperative blood flow evaluation of the reconstructive colon with ICG-FA is a promising method to reduce AL in laparoscopic rectal surgery. Since then, we have performed it in all patients as a routine procedure except for those with contraindications to ICG, such as drug allergy.
Oxygen saturation imaging technology
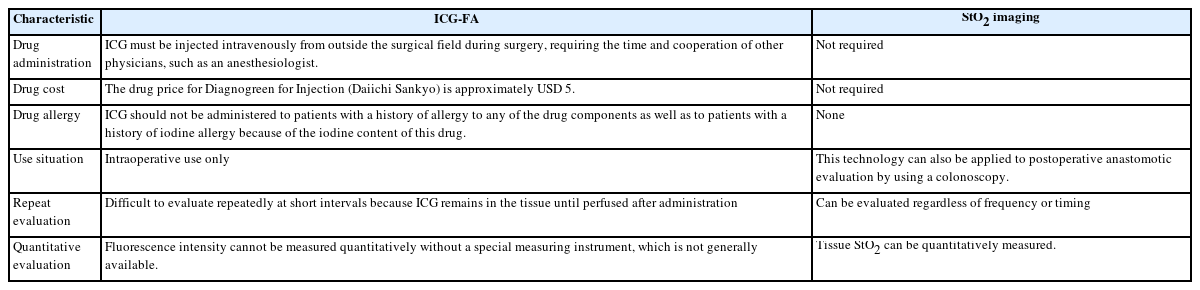
Oxygen saturation imaging is a technology for imaging oxygen saturation based on the difference in the absorption coefficient between oxidized hemoglobin (HbO2) and reduced hemoglobin (Hb) in the blood when the tissue is illuminated with a specific wavelength of light [31]. The blood flow evaluation method using this technology has the characteristics presented in Table 2 and is considered a promising method that can overcome the limitations of ICG-FA.
CONCLUSION
AL after rectal cancer surgery is not only a serious life-threatening complication but is also closely associated with deteriorated postoperative anorectal function and an increased local recurrence rate, thus causing significant disadvantages to patients. This article introduced the different anastomotic techniques in rectal surgery. We hope that it will promote an understanding of the appropriate anastomotic techniques and the importance of blood flow evaluation for the reconstructive colon, thereby reducing the incidence of AL after rectal surgery.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
None.
AUTHOR CONTRIBUTIONS
Conceptualization: all authors; Data curation: DM; Formal analysis: DM; Investigation: DM; Methodology: DM; Project administration: MI; Resources: MI; Supervision: MI; Visualization: DM; Writing–original draft: DM; Writing–review & editing: all authors. All authors have read and approved the final manuscript.