A comparative study of the pathological outcomes of robot-assisted versus open surgery for rectal cancer
Article information
Abstract
Purpose
The use of robot-assisted surgery for rectal cancer is increasing, but the pathological outcomes have not been fully clarified. We compared the surgical and pathological outcomes between robot-assisted and open surgery in specimens from patients operated on for rectal cancer.
Methods
All patients who underwent resection for rectal cancer from 2016 to 2018 were included (n=137). Specimens were divided into 3 sections to analyze the pathology of the lymph nodes.
Results
The total specimen lengths were shorter in the robot-assisted group than in the open surgery group (mean±standard deviation: 29.1±8.6 cm vs. 33.8±9.9 cm, P=0.004) because of a shorter proximal resection margin (21.7±8.7 cm vs. 26.4±10.6 cm, P=0.006). The number of recruited lymph nodes (35.8±21.8 vs. 39.6±16.5, P=0.604) and arterial vessel length (8.84±2.6 cm vs. 8.78±2.4 cm, P=0.891) did not differ significantly between the 2 surgical approaches. Lymph node metastases were found in 33 of 137 samples (24.1%), but the numbers did not differ significantly between the procedures. Among these 33 cases, metastatic lymph nodes were located in the mesorectum (75.8%), in the sigmoid colon mesentery (33.3%), and at the arterial ligation site of the inferior mesenteric artery (12.1%). The circumferential resection margin and the proportion of complete mesorectal fascia were comparable between the groups.
Conclusion
There were no significant differences between the 2 surgical approaches regarding arterial vessel length, recruitment of lymph node metastases, and resection margins.
INTRODUCTION
In recent years, minimally invasive surgical techniques have been developed for treating patients with colorectal cancer. Laparoscopic resection of colon cancer has become the standard technique worldwide because it has better short-term outcomes and similar oncological measures of success compared with open surgery. However, the results from randomized trials of laparoscopic surgery for rectal cancer have not conclusively proven this approach to be more beneficial than the open technique [1–3]. In contrast, some studies have shown that laparoscopic resection has a higher risk of achieving incomplete mesorectal excision [4] and a positive circumferential resection margin (CRM) [5, 6], presumably because of anatomical constraints in the pelvis, as well as ergonomic and optical limitations.
By providing a 3-dimensional view, better ambidextrous capability, and a stable camera platform, the robot-assisted approach maintains the advantages of a laparoscopic approach while overcoming its limitations [7]. The disadvantages of the technique are its high cost and long setup and procedural times [8, 9]. Studies have also shown improved postoperative recovery for robot-assisted surgery, as for laparoscopic surgery [10, 11], but no significant differences (or benefits) in oncological outcomes compared with open surgery have been reported [12, 13].
The surgical dissection techniques used in robot-assisted and laparoscopic surgery differ from those used in open surgery. The dissection and division of the tumor-feeding artery are most often performed medially in robot-assisted and laparoscopic surgery, but bilaterally in the open technique. Some studies have shown that the surgical dissection margins also differ between techniques, with longer distal margins obtained in the robot-assisted technique than in the laparoscopic and open techniques [14, 15].
The primary aim of the present study was to compare the surgical and pathological outcomes between robot-assisted and open surgery, including proximal and peripheral arterial ligation sites, arterial vessel and bowel length, the recruitment of lymph nodes, and the location of lymph node metastases in specimens from patients operated on for rectal cancer.
METHODS
Ethics statement
The study protocol was approved by the Regional Ethics Committee of Uppsala University, with a waiver for informed consent (No. 2013/099 and 2020/00426). The study was registered on ClinicalTrials.gov (identifier: NCT03314961).
Study population
This was a population-based cross-sectional study. All patients who underwent primary surgery for rectal adenocarcinomas from January 2016 to December 2018 at Västmanland Hospital Västerås (Västerås, Sweden) were enrolled. This hospital has a primary catchment population of 275,000 persons and is the only hospital treating rectal cancer in this well-defined geographical area. During this period, 205 patients were diagnosed with rectal cancer. The definition of rectal cancer was cancer with a distal margin 15 cm or less from the anal verge. Of the 205 consecutively enrolled patients, 65 were excluded because of disseminated disease and severe comorbidity, endoscopically removed polyp-type cancers, or pathologically complete responses after neoadjuvant treatment. One patient had total spontaneous remission without treatment (a hepatoid adenocarcinoma). Four patients were converted from robot-assisted to open surgery, and their specimens were included in the latter group for analysis due to late conversions. The study cohort of 137 patients who were operated on for rectal cancer with curative intent is presented in Fig. 1.

CONSORT (Consolidated Standards of Reporting Trials) flowchart of the selection of patients with rectal cancer during 2016–2018.
Data on patient characteristics including age, sex, American Society of Anesthesiologists (ASA) physical status (PS) classification, body mass index (BMI), tumor location, preoperative tumor stage, preoperative neoadjuvant treatment, and surgery type were collected from medical reports and the Swedish Colorectal Cancer Registry [16]. All patients underwent preoperative examinations with a computed tomography scan of the chest and abdomen, magnetic resonance imaging of the rectum, and an endoscopy with biopsy to confirm the diagnosis.
Neoadjuvant treatment and surgery
The tumor stage and grade were based on the TNM Classification of Malignant Tumours, 7th Edition [17] criteria. The tumors were staged preoperatively using the radiological tumor/node (rTN) system. Less advanced tumors (rT1–rT3bN0) with a low risk for local recurrence underwent no neoadjuvant radiochemotherapy. More advanced tumors (rT3 c/d N1/N2) with an intermediate risk were treated with short-course radiotherapy (25 Gy) with either direct or delayed surgery after 6 to 8 weeks. Advanced tumors (rT3–4) with a high risk, growth, or lymph node metastases, close to or outside the mesorectal fascia (MRF) received neoadjuvant either short-course (25 Gy) or long-course (50 Gy) radiotherapy with concurrent chemotherapy if there was no severe comorbidity.
The surgical technique was standardized, and total mesorectal excision (TME) was performed by 3 colorectal surgeons using open surgery and by 2 of them using robot-assisted surgery. Patients with better radiological TN stages underwent robot-assisted laparoscopic TME. More advanced MRF-positive tumors and tumors with growth close to the MRF or with suspected lateral lymph node metastases were assigned to the open surgery group, as a precautionary measure (since the pathological outcomes after laparoscopic surgery at the start of the study were unclear). However, some proximal and easily accessible T4 tumors were also selected for robotic surgery.
Robotic surgery was performed using the da Vinci Surgical System (Intuitive Surgical). In this procedure, the inferior mesenteric artery (IMA) was divided either centrally or peripherally at the superior rectal artery (SRA) close to the left colic artery (LCA) in a medial-to-lateral dissection. In the open technique, the sigmoid mesentery was first dissected, and the vessels were then divided. In both techniques, the level of ligation of the IMA was either 1 to 2 cm from the aorta for central ligations or at the SRA, very close to the origin of the LCA, for peripheral ligations (Fig. 2), depending on comorbidities and the tumor distance from the anal verge. We resected visible lymph nodes around IMA but did not perform D3 lymph node dissection in either group.
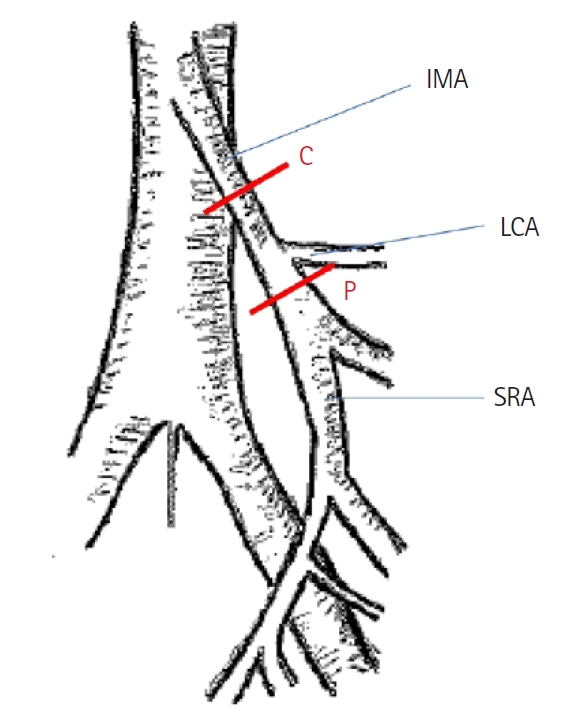
Anatomical location of central (C) and peripheral (P) arterial ligation of the tumor-feeding vessel, inferior mesenteric artery (IMA), superior rectal artery (SRA), and distal to the left colic artery (LCA).
In both groups, the splenic flexure was mobilized when necessary. The proximal resection margin was decided based on the level of the arterial ligation site, and the bowel was transected at the proximal sigmoid or descending colon depending on the arterial circulation. With the robotic technique, the specimen was taken out through a Pfannenstiel incision, and the bowel was thereafter divided. The intracorporal anastomosis was performed using laparoscopic instruments. Most low tumors (located <6 cm from the anal verge) required abdominoperineal resection (APR). Mid-located tumors (6–10 cm) or higher tumors (11–15 cm) had anterior resection (AR) or Hartmann resection (HR). All patients underwent perioperative rectal washout. Surgery time and estimated blood loss were recorded.
Analysis of pathology
The specimens were pinned on a cork plate and fixed for 72 to 96 hours in formaldehyde. Two trained gastrointestinal pathologists examined all gross and microscopic pathological findings. The specimen bowel length and the proximal and distal bowel margin from the tumor were measured. A detailed protocol was used in which the specimens were divided into 3 sections [18]. The completeness of the MRF was noted. The mesorectal fat, mesenteric fat from the sigmoid colon, and fat within 3 cm of the ligature of the IMA or proximal SRA were analyzed separately for lymph node retrieval. The fat-clearing technique was used to retrieve them. The length of the artery was measured from the arterial ligation to the bowel wall with a ruler along the main artery. The pathologists did not have prior information about the surgeons’ reported site of arterial ligation of the vessel.
All visible and palpable lymph nodes were harvested. They were stained with hematoxylin-eosin, cut into 2 sections, and examined for the presence of tumor metastases under light microscopy. Tumor size, location, extent (pT stage), lymph node involvement (pN stage), the number of lymph nodes, and degree of tumor differentiation, as well as invasion of lymphatic vessels, veins, or nerves, were recorded. Tumor or lymph node metastases in the CRM or within 1 mm from the resection margin were defined as positive margin. The incidence rate of complete MRF was noted.
Statistical analysis
Dichotomous data are presented as the frequency (%) or number (%) when appropriate. Continuous data are presented as the mean and standard deviation, supplemented with the median (range). Categorical variables were compared using the Pearson chi-square test or Fisher exact test. The significance level of all tests was set at P<0.05. All statistical analyses were performed using IBM SPSS Statistics ver. 26 (IBM Corp).
RESULTS
The demographic factors, tumor type, and treatment characteristics of the 137 patients operated on for rectal cancer from 2016 to 2018 are presented in Table 1. The patients in the robot-assisted surgery group were older and had less advanced T and N tumor categories. The 2 groups were similar with regard to sex, BMI, ASA PS grade, and type of resection performed. Seventy-five patients (54.7%) underwent a sphincter-preserving AR; 51 (37.2%) received APR; and 11 (8.0%) underwent HR. The distribution of procedures did not differ significantly between the robot-assisted and open-surgery groups (P=0.104) (Table 2). The operation time was longer for robot-assisted surgery, and blood loss was greater for open surgery with AR and APR. Central ligation of the IMA was reported in 107 operations (78.1%), and the rate of this type of ligation did not differ significantly between the groups (Table 3). Arterial vessel length did not substantially differ between patients who received central ligation of the IMA and those who received ligation of the SRA close to the LCA.
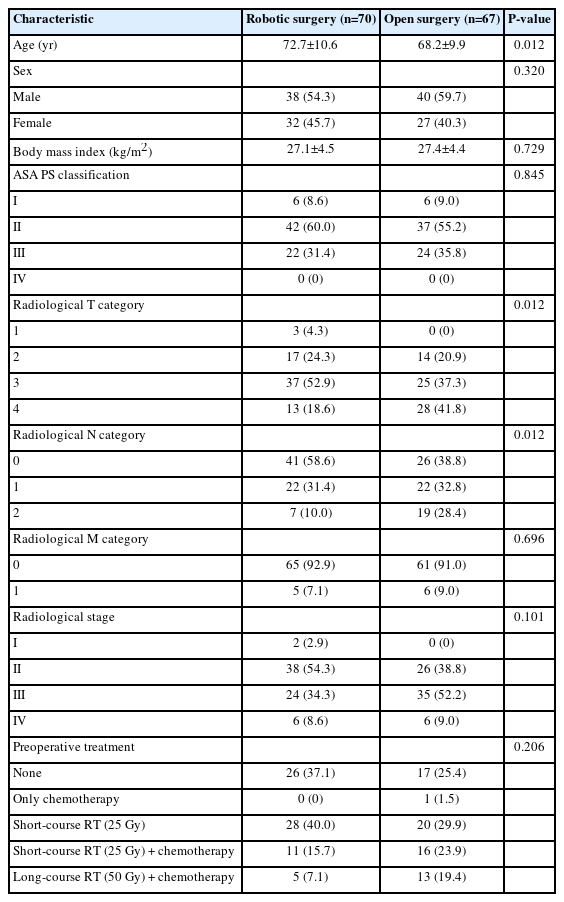
Demographic, tumor, and treatment characteristics of patients with rectal cancer who underwent curative surgery (n=137)
The mean of bowel specimen length was shorter for robot-assisted than open surgery (29.1±8.6 cm vs. 33.8±9.9 cm, P=0.004); therefore, the proximal bowel resection margin in relation to the tumor was shorter (Table 3). No patient had a positive CRM or an intraoperative perforation. The mean tumor size (measured as a length in centimeters) was not related to tumor stage and did not differ significantly according to the type of surgery (robot-assisted, 3.0 cm [range, 1–6 cm]; open surgery, 3.5 cm [range, 1–19]). The overall median number of recruited lymph nodes was 35 (range, 5–132). The distribution of lymph nodes did not differ between robot-assisted and open surgery for the mesorectum, sigmoid mesentery, or arterial ligation site (Table 3).
Rectal cancer specimens from 33 patients (24.1%) had metastatic lymph nodes. In 25 of these specimens (75.8%), the metastatic lymph nodes were located in the mesorectum. Eleven of the 33 specimens (33.3%) also had metastatic lymph nodes in the sigmoid colon mesentery. In 6 of the specimens (18.2%), metastatic lymph nodes were found only in this mesentery. Four of the 6 patients had received neoadjuvant chemoradiotherapy, with no significant difference between the 2 groups.
Four of the patients had lymph node metastases close to the arterial ligation; 3 were at the ligation of the IMA and 1 was at the ligation of the SRA. Three of these patients had robot-assisted surgery and 1 underwent open surgery.
Thirty-three specimens (24.1%) had tumor deposits, 3 of which were without positive lymph nodes. Invasion was detected in lymph vessels in 41 specimens (29.9%), veins in 54 (39.4%), and nerves in 25 (18.2%), but these rates did not differ between groups. Of the 4 patients who exhibited a complete pathological tumor response (yT0), 1 had lymph node metastases in the sigmoid mesentery.
DISCUSSION
In this comparative study of robot-assisted versus open surgery in patients with rectal cancer, an analysis of the specimens’ pathology showed that the arterial vessel length, total number of retrieved lymph nodes, and lymph node metastases did not differ significantly between the 2 types of surgical approach. There were also no differences in radicality concerning CRM measures and the very high rate of complete MRF assessed by the pathologists. The proximal bowel lengths were shorter after robot-assisted surgery, probably because the specimen was taken out through a Pfannenstiel incision, but there were no substantial differences between the important distal bowel lengths.
Since improvements in the surgical technique with TME for rectal cancer were reported in 1986 [19], further developments have included the use of a laparoscopic technique for colorectal surgery, which has now gained greater acceptance. Laparoscopic surgery for rectal cancer has been proven to have advantages in terms of less postoperative pain and a shorter postoperative recovery [1]. However, because it could be more technically demanding, some early studies have shown that laparoscopic surgery has not been able to meet the demand for the high standards set for TME in rectal cancer surgery [14]. By contrast, robot-assisted surgery has the potential to achieve better mesorectal dissection with better visualization and opportunity to preserve the autonomic nerves [20]. However, given the limitations of robot-assisted techniques in terms of tactile and digital examinations and the risk of unforced stretching or harm caused by the instruments in MRF-positive tumors or in patients with lymph node metastases outside or close to the MRF, patients with these conditions were operated on using the open technique in the present study. Thus, older patients were more likely to receive robot-assisted surgery, and the tumor stages were less advanced in these patients.
There is an ongoing debate about the optimal level of arterial ligation around the IMA in rectal cancer surgery. In our previous study on the localization of mesenteric lymph node metastases in open standardized TME surgery for rectal cancer, we did not find a benefit of central ligation of the IMA versus ligation close to the origin of the LCA in terms of the recruitment of lymph node metastases [18]. In both of these studies, the vessel lengths were similar, thanks to a standardized surgical technique with the 2 types of ligatures close to the origin of the LCA. Furthermore, in our previously published paper on open surgery, no lymph node metastases were found at the arterial ligation site [18]. In the present study, 4 patients had lymph node metastases at the arterial ligation site; these patients had advanced tumor stages and received neoadjuvant treatment. The rationale for this difference is unclear because the vessel lengths were similar in both groups.
Another finding in this study, consistent with our previous report [18] was that lymph node metastases in 6 patients were only found in the sigmoid mesentery. For this reason, it is important not to divide the proximal bowel and sigmoid mesentery too close to the tumor. All of these patients received neoadjuvant radiotherapy, which might explain the lymph node metastasis negativity in the mesorectum.
Other published data have not shown a survival benefit for central versus peripheral arterial ligation of the IMA. By contrast, an increased risk for anastomotic leakage and urethral dysfunction has been reported [21], but studies are inconclusive.
The strengths of this study are that 3 experienced surgeons performed the operations and that 2 qualified pathologists analyzed the specimens without knowing the surgeons’ reported choice of ligature site. We found similar data on robot-assisted surgery as in our previous paper [18], which included only an analysis of specimens from open surgery. As far as we know, this is the first study to compare pathological outcomes regarding the site of arterial ligation, arterial vessel, and bowel lengths in specimens from robot-assisted and open TME rectal cancer surgery.
The limitations of this study are that it was a single-center study with small sample size; patients with better radiological TN stages and MRF-negative tumors were selected for robot-assisted surgery, which made the groups not fully comparable; and there was not a distinct selection protocol for robot-assisted surgery. However, the groups were similar with regard to sex, BMI, ASA PS grade, and type of resections performed. Since we followed the National Swedish guidelines, a very high proportion of patients—75% and 63% in the open and robot-assisted surgery groups, respectively—received radiotherapy. This could also have affected the pathological outcomes. The motivation for the choice of central or peripheral arterial ligation was not recorded in the study protocol, but in the medical records. Most patients with AR had a central arterial ligation (71 of 75, 94.7%). Patients with a high level of comorbidities (ASA PS grade III) and tumors close to the anal verge more often had a peripheral ligature (data not shown). We think that the difference in operation time was a learning effect in the beginning and the difference in bleeding amount was due to the use of advanced bipolar device in robot-assisted and monopolar diathermy in open surgery.
In conclusion, we found no differences between techniques regarding arterial vessel length, distal bowel length, recruitment of lymph nodes, lymph node metastases, or radicality as judged by CRM and completeness of the mesorectal excision, although the 2 groups were not fully comparable. Randomized studies are needed to clarify whether the robot-assisted technique is pathologically and oncologically safe for advanced T4 tumors.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Acknowledgments
The authors thank the staff at the Department of Pathology, Västmanland Hospital Västerås (Västerås, Sweden), for their help with the pathology analyses, and Maziar Nikberg and Abbas Chabok for performing surgery at the Department of Surgery, Västmanland Hospital Västerås.
Author contributions
Conceptualization: CT; Data curation: RR; Investigation: CK, KS; Methodology: CK, KS, CT; Project administration: RR, CT; Software: RR; Supervision: CT; Writing–original draft: RR, CK, CT; Writing–review & editing: KS, CT. All authors read and approved the final manuscript.


