Outcomes of redo for failed colorectal or coloanal anastomoses: a systematic review and meta-analysis
Article information
Abstract
Purpose
This study aimed to review the outcomes of redo procedures for failed colorectal or coloanal anastomoses.
Methods
A systematic review was performed using the PubMed, Embase, Cochrane, and LILACS databases. The inclusion criteria were adult patients undergoing colectomy with primary colorectal or coloanal anastomosis and studies that assessed the postoperative results. The protocol is registered in PROSPERO (No. CRD42021267715).
Results
Eleven articles met the eligibility criteria and were selected. The studied population size ranged from 7 to 78 patients. The overall mortality rate was 0% (95% confidence interval [CI], 0%–0.01%). The postoperative complication rate was 40% (95% CI, 40%–50%). The length of hospital stay was 13.68 days (95% CI, 11.3–16.06 days). After redo surgery, 82% of the patients were free of stoma (95% CI, 75%–90%), and 24% of patients (95% CI, 0%–39%) had fecal incontinence. Neoadjuvant chemoradiotherapy (P=0.002) was associated with a lower probability of being free of stoma in meta-regression.
Conclusion
Redo colorectal and coloanal anastomoses are strategies to restore colonic continuity. The decision to perform a redo operation should be based on a proper evaluation of the morbidity and mortality risks, the probability of remaining free of stoma, the quality of life, and a functional assessment.
INTRODUCTION
Colorectal and coloanal anastomoses are technically demanding, and the risk of complications, such as leakage or stenosis, is around 20% [1]. Furthermore, a substantial proportion of these leaks never heal [1]. An unhealed anastomosis can result in a complex infection inside and outside the pelvis, which is often the reason for never closing a stoma [2]. Permanent stomas lead to a deterioration of the quality of life and self-esteem, and distortion of body image [3]. Consequently, preventing the patient from having a permanent colostomy or ileostomy is one of the main goals of colorectal surgery.
Redo surgery is often performed to restore bowel continuity and thereby prevent a definitive colostomy. However, intestinal functional outcomes are as important as the restoration of bowel continuity [4]. Redo anastomosis can be indicated due to rectovaginal fistula, anastomotic stricture, dehiscence, and chronic pelvic sepsis. The main techniques are immediate colorectal or coloanal anastomosis and delayed coloanal anastomosis (pull-through) [5].
Nonetheless, there remains a lack of consensus regarding redo surgery after colorectal and coloanal anastomosis failure. Thus, this study aimed to review the literature to assess the postoperative results, the success rate (restoration of digestive continuity), and the functional results of redo surgery.
METHODS
Study design
This research followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) precepts. The research protocol is registered in the PROSPERO (International Prospective Register of Systematic Reviews) database (No. CRD4202126-7715). To carry out the systematic review and meta-analysis, the following steps were followed. Evidence was selected based on the inclusion and exclusion criteria. The inclusion criteria were the following: adults (>18 years old); patients undergoing colectomy with primary, colorectal, or coloanal anastomosis; studies that assessed postoperative results in the short or long term; and observational or interventional studies. The exclusion criteria were the following: studies in animal models; letters, editorials, conference abstracts, and guidelines; and full-text unavailable studies. When more than 1 study with the same population was identified, the most complete study was included. There was no restriction on the search period or language.
Search
The search for evidence was performed in the PubMed, Embase, Cochrane (CENTRAL), and LILACS databases. Articles were selected manually according to the predefined eligibility criteria. Two independent authors (RPG and GAA) screened the literature. Any disagreement about the inclusion of a study was resolved by discussion. A third senior author (FT) acted as the final arbiter if consensus was not reached. The following search strategy was used (Supplementary Material 1): (colorectal OR rectal OR rectum) AND (neoplasm OR cancer OR tumor) AND (surgery OR resection OR colectomy OR rectosigmoidectomy OR proctocolectomy) AND (redo OR re-do OR reoperation).
Certainty assessment and risk of bias assessment
The evaluation of certainty was done through GRADE (Grading of Recommendations, Assessment, Development and Evaluations). The ROBINS-I (Risk Of Bias in Non-randomized Studies-of Interventions) tool was used to assess bias.
Data extraction
Data was extracted following pre-determined eligibility criteria: (1) general information (authors, year of publication, and title); (2) characteristics of the patients and neoplasms (sample size, age, sex, follow-up time, and indication for colectomy); (3) interventions (surgical access and neoadjuvant therapy); and (4) outcomes (postoperative complications, length of hospital stay [LOS], surgical technique, and postoperative mortality).
Statistical analysis
The absolute numbers were extracted and analyzed with Stata ver. 16.0 (StataCorp). The extracted results were evaluated through meta-analyses. The event rate and the corresponding 95% confidence intervals (CIs) were provided. A random-effects analysis model was applied to adjust for expected heterogeneity between studies.
We performed meta-regression on study-level summary data using the “metareg” command. Meta-regression was used to identify covariates that could influence the outcomes. We also performed subgroup analysis according to the surgical redo technique (traditional redo anastomosis vs. the pull-through technique).
RESULTS
Baseline characteristics of the included studies
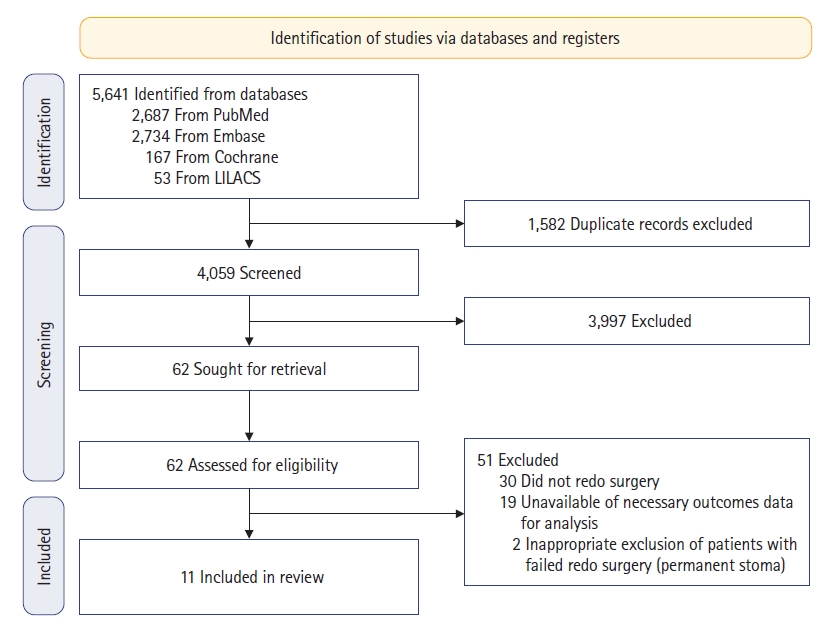
We initially identified a total of 5,641 articles in the databases, and after removing duplicates, 4,059 were screened. Of those articles, 11 [5–15] met all the eligibility criteria and were selected (Fig. 1).
Only observational studies were identified. The studied population size ranged from 7 to 78 patients. The mean age of participants ranged from 51 to 62 years. The mean follow-up ranged from 11 to 37 months for outcomes. Seven studies [5, 6, 8, 9, 12–14] included both malignant and benign conditions, 3 [7, 10, 15] included only malignant diagnoses, and 1 [11] did not specify this information. Neoadjuvant therapy was performed in 7 studies [5, 8, 10, 12–15], with proportions ranging from 15% to 71%. The pull-through technique was performed in 4 studies [5, 6, 10, 12]. The baseline characteristics of the included studies are reported in Table 1 [5–15].
Postoperative mortality
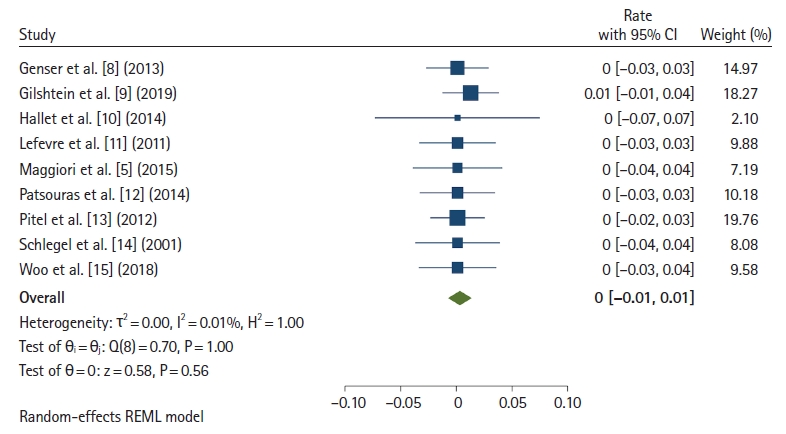
Postoperative mortality was reported in 9 studies [5, 8–15]. Fig. 2 [5, 8–15] shows the mortality rate of redo surgery. The overall mortality rate was 0% (95% CI, 0%–0.01%; I2=0.01%). In the subgroup analysis, the mortality rate of the pull-through technique was 0% (95% CI, 0%–0.03%; I2=0.01%), and that of traditional redo anastomosis (immediate coloanal anastomosis) rate was 0% (95% CI, 0%–0.02%; I2=0.01%).
Postoperative complications
Postoperative complications were reported in 9 studies (Fig. 3) [5, 8–15]. The postoperative complications rate was 40% (95% CI, 40%–50%; I2=78.47%). The postoperative complications comprised peritonitis, necrosis, ileum, ileal fistula, incisional hernia, pelvic abscess, urinary infection, ureter lesion, hematoma, wound infection, and anastomotic leak. In the subgroup analysis, the postoperative complication rate of the pull-through technique was 51% (95% CI, 35%–65%; I2=25.53%), and that of immediate coloanal anastomosis was 35% (95% CI, 22%–47%; I2=82.57%).
Length of hospital stay
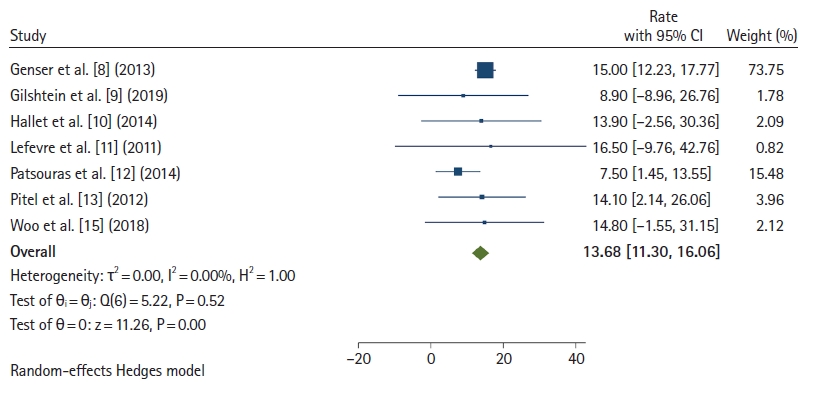
The LOS was reported in 7 studies (Fig. 4) [8–13, 15]. The mean LOS was 13.68 days (95% CI, 11.30–16.06 days; I2=0). The pull-through subgroup showed an average LOS of 8.26 days (95% CI, 2.58–13.94 days), while the immediate coloanal anastomosis technique had an average LOS of 14.84 days (95% CI, 12.21–17.46 days).
Incontinence
Incontinence after redo surgery was reported in 9 studies [5–8, 11–15]. Fig. 5 [5–8, 11–15] shows that 24% of patients (95% CI, 10%–39%; I2=93.64%) had fecal incontinence after redo surgery. The incontinence rate in the pull-through subgroup was 18% (95% CI, 10%–26%), and that in the immediate coloanal anastomosis group was 28% (95% CI, 10%–47%).
Freedom from stoma at the end of follow-up
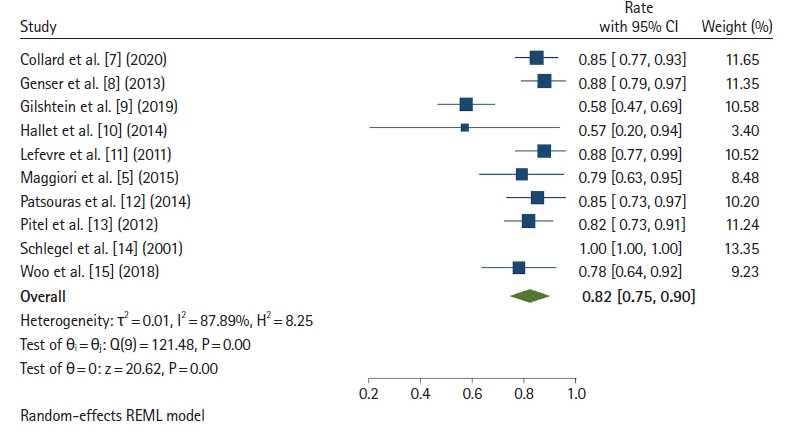
Ten studies [5, 7–15] reported the proportion of patients free of stoma at the end of follow-up. Fig. 6 [5, 7–15] shows that 82% of patients were free of stoma after redo surgery (95% CI, 75%–90%; I2=87.89%). The rate of patients free of stoma for the pull-through technique was 81% (95% CI, 72%–91%), and that of immediate coloanal anastomosis was 83% (95% CI, 73%–93%).
Quality of life evaluation
Three studies [6, 7, 13] evaluated the quality of life. However, since the studies’ endpoints were not comparable, a quantitative synthesis was not possible. Only a qualitative synthesis was performed.
Pitel et al. [13] evaluated the quality of life in 46 patients and functional results in 43. The 12-Item Short Form Health Survey (SF-12) scores of patients who underwent redo coloanal anastomosis were similar to the SF-12 scores of the general population. Twenty-seven patients (62.8%) complained of fragmentation of stools. Eighteen patients (41.9%) took medication for transit control.
In the article of Collard et al. [7], 62 patients responded to the low anterior rectal syndrome (LARS) questionnaire and 60 (96.8%) completed the Gastrointestinal Quality of Life Index (GIQLI) score, with a median interval after redo surgery of 69 months (interquartile range [IQR], 38–100 months). The median value of the GIQLI score was 110 (IQR, 100–120). In the univariate analysis conducted to identify factors impacting the long-term quality of life, only the absence of major LARS was significantly associated with a good quality of life. Poor quality of life (GIQLI of < 110) was not significantly associated with sexual and urinary dysfunction in men or women.
Boullenois et al. [6] evaluated 3 groups: delayed coloanal anastomosis (group A); immediate coloanal anastomosis (group B); and a control group composed of patients who underwent primary anterior resection with coloanal anastomosis (group C). Group C had the higher LARS and GIQLI scores; 19 (IQR, 15–32) and 12 (IQR, 108–144), respectively. Tendencies for worse outcomes were noted in group A than in group C (LARS, P=0.057; GIQLI, P=0.066). In the redo surgery groups, the LARS and GIQLI scores did not differ significantly between groups A and B (P=0.292 and P=0.728, respectively).
Meta-regression
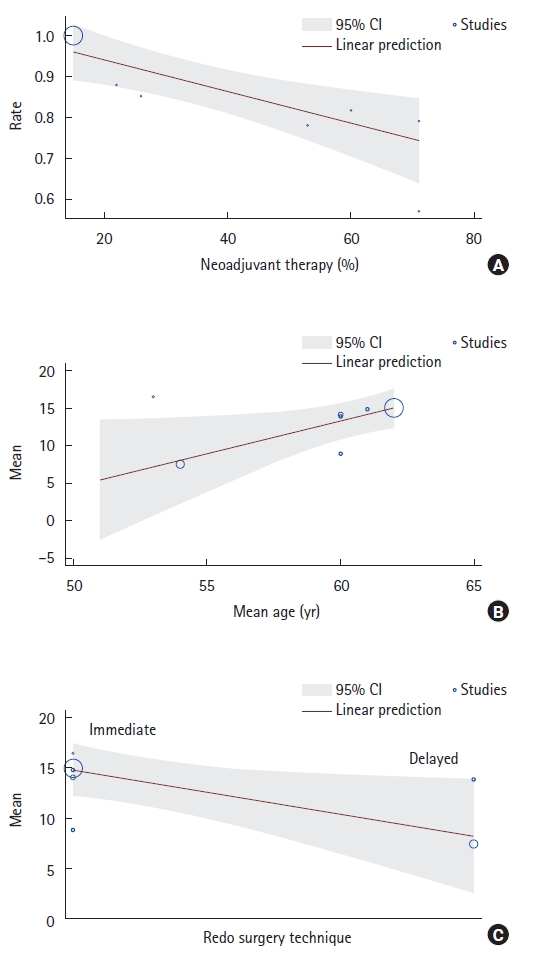
Meta-regression was used to identify covariates that could influence the outcomes (Table 2). We found that neoadjuvant chemoradiotherapy (P=0.002) was associated with a lower probability of being free of stoma at the end of follow-up. Furthermore, age (P=0.035) was associated with prolonged LOS, and the pull-through technique (P=0.039) was associated with lower LOS (Fig. 7).
Risk of bias assessment
To assess biases, the ROBINS-I tool was used. All articles were considered to have a critical overall risk of bias, mostly because of the risk of selection bias, the risk of bias due to confounding, and the risk of bias in the classification of interventions (Supplementary Table 1).
Certainty assessment
We analyzed the certainty with GRADE, and the overall certainty of evidence ranged from very low to moderate (Supplementary Fig. 1).
DISCUSSION
The present systematic review and meta-analysis showed that redo of colorectal anastomosis has low mortality. However, at the same time, the complexity of this surgical procedure makes patients susceptible to high morbidity, and even after redo surgery, there is the risk of permanent stoma or incontinence.
Redo colorectal anastomosis is associated with a high risk of morbidity. The postoperative complication rate was 40% (95% CI, 40%–50%). However, keeping a permanent stoma also has inherent problems. Colostomy, temporary or permanent, can have a serious negative impact on the quality of life of patients with colorectal cancer, as these patients have physical and psychological limitations [16]. Almost 85% of patients with definitive stomas reported that they were not happy with their quality of life. Stomal complications are found in approximately 60% of patients with a permanent stoma, including skin irritation, plain stoma, parastomal hernia, stoma retraction, dehydration, renal insufficiency, and electrolyte disorders [17].
The main goal of redo surgery is for the patient to be stoma-free. However, some patients who underwent redo surgery ended up with a stoma. Approximately 82% of patients were free of stoma in this study after the redo procedure. Consequently, patients should be aware of the possibility of the failure of redo surgery, and the decision to undergo a new procedure should be shared between patients and clinicians. Variables associated with poorer outcomes should be pondered when making this decision.
Patients who underwent neoadjuvant chemoradiotherapy had a lower chance of closure, as seen in the meta-regression. Pelvic radiation affects normal tissue, creating a hostile environment for surgical dissection. den Dulk et al. [18] showed that preoperative radiotherapy was significantly associated with a decreased likelihood of stoma reversal. Furthermore, they found that older age, secondary stoma construction, an end colostomy or ileostomy, and any postoperative complication were limiting factors for stoma reversal.
As shown in the meta-regression, older patients had longer hospital stays. This result could be explained by the fact that postoperative morbidity is more common in older patients. Bahrmann et al. [19] showed that older patients presenting to the emergency room had a high rate of comorbid conditions (> 2 comorbid conditions), and it is known that Charlson Comorbidity Index and the Barthel Index independently predict the LOS.
Restoring intestinal continuity is not enough to ensure quality of life. After redo surgery, 24% of patients had a significant complaint of fecal incontinence. Herrle et al. [20] showed that the median Wexner score for fecal continence at 6 months after stoma closure was 10 (range, 0–20). On average, more than half of all patients had a severe or very severe impairment of fecal continence, showing that being free of stoma is not synonymous with a good quality of life.
Patients need to be aware of the risk of incontinence, and the decision to perform redo surgery should be shared with the patient. Multi-professional work with a physiotherapy team for pelvic physiotherapy is essential. In addition, doctors should consider the patient’s previous history of incontinence or other signs that may increase the risk of incontinence, such as multiple births and older age [21, 22]. Biofeedback is an active patient reeducation technique that uses a device that records and amplifies the activity practiced by the patient with no electrical stimulation [23]. After a 4-month physiotherapy treatment program based on pelvic floor exercises and biofeedback, 83% of patients reported improved quality of life, and 75% reported reduced symptoms [24].
Meta-regression suggested that the choice of the redo technique could influence the LOS. The pull-through subgroup showed an average LOS of 8.26 days, while that of the traditional colorectal and coloanal anastomosis technique was 14.84 days. The main advantage of the pull-through procedure is that it avoids pelvic dissection, reducing the risks of locoregional complications in a hostile pelvis, which could negatively impact LOS [12]. However, it is important to note that no direct comparisons between the traditional redo anastomosis and the pull-through technique were performed, and only future controlled trials will determine the role of each surgical redo technique.
Two other systematic reviews [25, 26] evaluated redo anastomosis. Halkias et al. [25] performed a systematic review without meta-analysis and evaluated only reoperative laparoscopic surgery. Fransvea et al. [26] investigated the outcomes of laparoscopic redo management of early postoperative complications following laparoscopic colorectal surgery. The authors focused on the LOS, morbidity, and mortality. Neither Halkias et al. [25] nor Fransvea et al. [26] investigated the incontinence rate or the probability of being free of stoma after redo surgery. Our study investigated redo surgery after colorectal resection, both by the traditional immediate redo procedure and the pull-through technique. Besides the early postoperative outcomes, we also investigated fecal incontinence, quality of life, and the stoma freedom rate. None of the previous systematic reviews performed a meta-regression investigating covariates that could possibly influence the outcomes.
The main limitations of the present review were related to the quality of evidence of the included studies. All studies were observational, with small sample sizes, significant interstudy clinical variability, a high risk of bias, and a low degree of certainty. Examining uncertainty is always a complex task in these situations, and sensitivity analysis models may fail to depict the real uncertainty. Consequently, we chose not to conduct sensitivity analyses and assumed the uncertainty as an inherent weakness of this manuscript. New studies should be proposed in this area, especially clinical trials with a large number of patients, evaluating the results of different surgical techniques and comparing the traditional immediate colorectal and coloanal anastomoses with the pull-through technique. Another point to be addressed in future studies would be the functional results after redo surgery, especially involving fecal incontinence. The quality of life after redo surgery was analyzed in the minority of the studies but should be included in future analyses. Another issue to be investigated in future trials is the optimal timing of redo surgery.
Redo colorectal and coloanal anastomoses are strategies to restore colonic continuity. The decision to perform redo surgery should be based on a proper evaluation of the morbidity and mortality risks, probability of staying free of stoma at the end of therapy, quality of life, and a functional assessment. Radiation therapy, age, and the surgical technique may influence the outcomes.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author contributions
Conceptualization: FT. Data curation: RPG, GAA. Formal analysis: FT. Investigation: RPG, GAA. Resources: LSG. Supervision, Validation: VES, SEAA. Visualization: RVP. Writing–original draft: LAS, RBRS. Writing–review & editing: all authors. All authors have read and approved the final manuscript.
Supplementary materials
Supplementary Material 1.
Study search strategy.
Supplementary Table 1.
Risk of bias assessment (ROBINS-I)
Supplementary Fig. 1.
Certainty assessment (GRADEpro).
Supplementary materials are available from https://doi.org/10.3393/ac.2022.00605.0086.