- Search
| Ann Coloproctol > Volume 39(3); 2023 > Article |
|
Abstract
Colitis caused by vasculitis is a rare and poorly understood pathology. Little evidence exists on its clinical presentation, path to diagnosis, and surgical management. In this report, we present a case report and literature review. A healthy 20-year-old male patient presented with hemorrhagic colitis requiring total colectomy with end ileostomy. Pathological examination showed pancolitis with multiple ulcers, transmural inflammation, hemorrhage, and microvascular thrombosis. Extensive serological testing revealed elevated cytoplasmic antineutrophil cytoplasmic antibody (c-ANCA) and eosinophilia, leading to a diagnosis of eosinophilic granulomatosis with polyangiitis (EGPA) and vasculitis-induced colitis. A literature review was subsequently conducted. Nineteen studies were found documenting vasculitis-induced colitis in the absence of inflammatory bowel disease (IBD). Systemic signs of vasculitis, hemorrhagic colitis, and progression to fulminant colitis were present. Of all patients, 40.0% required colorectal surgery and 62.5% of those patients received a stoma; 25% underwent emergency surgery following failed immunosuppression. All cases relied on clinical correlation with serology and/or histopathology to reach a final diagnosis. We report a case of vasculitis-induced colitis caused by c-ANCA–positive EGPA. The review shows that vasculitis-induced colitis without IBD is an important differential that clinicians should be aware of in patients presenting with colitis.
Colitis is inflammation of the colon, which can be acute, self-limiting, or chronic. Colitis can be caused by a number of conditions, which are broadly subtyped into autoimmune, infections, ischemic, iatrogenic (e.g., diversion or radiation colitis), or microscopic. Vasculitis can cause ischemic colitis due to the involvement of mesenteric arteries and veins (or even smaller vessels) [1].
Colitis is a core component of inflammatory bowel disease (IBD). IBD includes 2 conditions: ulcerative colitis (UC) and Crohn disease (CD). Particularly in UC, colitis can acutely manifest with fulminant colitis and toxic megacolon [2, 3]. An acute colitis flare is commonly managed with anti-inflammatory and immunosuppressive agents, with potential progression to surgical management if the disease progresses to fulminant colitis or toxic megacolon [4]. As IBD is believed to be an immune-mediated inflammatory disease, patients with IBD have an elevated risk of developing autoimmune diseases, including vasculitides [5]. Many studies have explored the propensity for vasculitides in IBD to cause colitis independently [6–9]. Behçet disease is another multisystemic inflammatory disease (albeit rarer), where intestinal manifestations of colitis are known to occur, and typically coincides with IBD [10]. Antineutrophil cytoplasmic antibody (ANCA) is an autoantibody directed against the constituents of neutrophil granulocytes. A distinction is made between cytoplasmic ANCA (c-ANCA) and perinuclear ANCA (p-ANCA), which can be present in IBD, vasculitis, or autoimmune disease, and can therefore be observed serologically in patients with colitis caused by one of these conditions [11].
Colitis caused by primary vasculitis in the absence of IBD, however, is a much rarer and poorly understood entity [1]. There is sparse literature documenting the presentation, diagnosis, and subsequent management of vasculitis-induced colitis. Furthermore, there are few studies to guide surgeons as to the optimal surgical management of this condition [1].
In this study, we present a case of c-ANCA–positive vasculitisinduced colitis requiring an extensive surgical intervention and conduct a review of the published literature documenting vasculitis-induced colitis in the absence of IBD.
An otherwise healthy 20-year-old male patient presented to a rural Australian emergency department with a 2-week history of rectal bleeding, diarrhea, and lower abdominal pain. His only past medical history was asthma managed by salbutamol intermittently as required. He took no other medications aside from salbutamol. An examination revealed a soft abdomen, with mild tenderness in the lower abdomen. He was treated with oral analgesia and was discharged home with a plan for outpatient colonoscopy.
The patient re-presented 4 days later with increasing rectal bleeding, hematuria, and right iliac fossa, rectal, and bilateral knee pain. A digital rectal examination revealed tenderness in the absence of anal fissures, perianal fistulae, or abscesses. A computed tomography (CT) scan of the abdomen revealed complete pancolitis, most marked in the sigmoid colon and rectum, with no evidence of perforation or abscess, as shown in Fig. 1. There was masked bowel wall thickening and edema involving the rectum and sigmoid colon, and to a lesser extent the large bowel. There was no evidence of free gas or free fluid, with preserved mucosal enhancement. Ischemic changes were seen in the sigmoid colon. All fecal microbial tests were negative. He was admitted under colorectal surgery and treated with intravenous antibiotics. Colonoscopy on day 2 reached the right colon and showed severe diffuse colitis throughout the entire colon, including the rectum. The colonic mucosa was erythematous and friable, suggesting ulcerative changes, as shown in Fig. 2. The endoscopic appearance was consistent with Mayo-3 (severe) UC [12]. There was contact mucosal bleeding alongside several petechiae. Intravenous hydrocortisone and infliximab were commenced on days 2 and 3, respectively. However, the patient’s condition did not improve. He developed worsening abdominal pain with declining hemoglobin (167–82 g/L), albumin (27–12 g/L), and platelet count (267× 109/L to 55× 109/L). An abdominal x-ray examination revealed dilatation of the caecum, raising concerns for toxic megacolon.
Due to failed medical therapy with an increasing white cell count (13.9× 109/L to 21.1× 109/L) and an increasing C-reactive protein level (132–254 mg/L), he underwent surgery on day 4, receiving a laparoscopic total colectomy with end ileostomy. The operation revealed florid colitis (ischemic and hemorrhagic), predominantly of the right and transverse colon, with patches of necrosis of the proximal transverse colon, as shown by the gross specimen in Fig. 3. Unusual bruising was present in the preperitoneal, retroperitoneal, and retrorectal spaces, but no hematoma was present. The patient’s rectosigmoid stump was implanted subcutaneously to minimize stump blowout. Postoperatively, he spent 2 days in the intensive care unit where he received total-parental nutrition. He was discharged home after 7 days in hospital.
Initial endoscopic biopsies (ascending, transverse, distal, descending, sigmoid colon, and rectum) revealed acute pancolitis, with attenuation of the surface epithelium, small granulomas, and mainly intact crypt architecture. No malignancy was seen. A histological examination of the total colectomy specimen, as shown in Fig. 3, revealed pancolitis with multiple ulcers and transmural inflammatory infiltrate, prominent hemorrhage, and widespread microvascular thrombosis. The features were suggestive of toxic megacolon, as a complication of vasculitis hypercoagulative disorder-induced colitis. The small bowel was normal on histopathology. The patient was referred to a metropolitan center for rheumatological testing, which revealed an elevated c-ANCA of 101 U/mL and mild eosinophilia (1.1 × 109/L). All other tests were negative (von Willebrand, factor V Leiden, prothrombin, thrombophilia screen, antinuclear antibodies [ANA], rheumatoid factor, p-ANCA, hepatitis B, hepatitis C, HIV, and tuberculosis). IBD was excluded as histological findings were inconsistent with typical features seen in UC or CD. Behçet disease was excluded as the patient did not have any core cutaneous features, which are required for a diagnosis according to the International Criteria for Behçet Disease [10]. The patient was diagnosed with eosinophilic granulomatosis with polyangiitis (EGPA) by rheumatology. It is believed that c-ANCA–positive EGPA vasculitis induced an episode of colitis that mimicked toxic colitis/megacolon, as commonly seen in acute UC. He was subsequently commenced on rituximab treatment. One month on, he is well and back to work with resolution of his intestinal and extraintestinal symptoms. He remains on life-long immunosuppressive treatment. His final diagnosis was vasculitis-induced colitis secondary to c-ANCA–positive EGPA.
Ethical approval was obtained from the Human Ethics Review Committee of Bendigo Health (No. LNR/65474/BHCG-2022-217859). Written consent was obtained from the patient for the publication of this case report. The review followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines and was prospectively registered with PROSPERO (No. CRD42022339409).
A literature search was performed in June 2022. The MEDLINE, Embase, and Scopus databases were searched for articles from 1980 onwards. The MeSH (Medical Subject Heading) search terms were (colitis) AND (vasculitis) AND (colorectal surgery). Conference abstracts, editorials, and literature reviews were excluded. Articles were limited to English. Vasculitis was defined according to the 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides [13]. Studies were excluded if they included pregnant or pediatric patients. References within and citations of all included articles were screened for completeness. The full texts were evaluated against predetermined inclusion criteria: (1) original research of (2) adult patients presenting with acute vasculitis-induced colitis without concomitant IBD who (3) received emergent management (either colorectal surgical intervention or medical management).
Titles, abstracts, and full texts were screened independently by 2 reviewers (JAP and CHAL) using the Covidence platform (Covidence). Disagreements were resolved by a third reviewer (GS). Data including study design, patient demographics, disease presentation, diagnosis, medical management, endoscopic and surgical interventions, and patient outcomes were extracted.
In total, 260 unique records were identified, of which 94 proceeded to full-text screening. Of these, 75 were excluded (35 studied patients with IBD, 22 studied systemic vasculitis without colitis, and others studied children and pregnant women). Nineteen studies with 20 patients were included in the final review. There were 18 case reports and 1 case series (see Fig. 4 for the PRISMA flowchart).
The average patient age was 49 years, and a half of the patients (10 of 20) were female (Table 1) [14–32]. No patient was reported to have colitis induced by large artery disease (giant cell arteritis, Takayasu arteritis, or isolated aortitis). Two studies reported medium-vessel vasculitis polyarteritis nodosa [14, 24]. No reports of medium-vessel vasculitis or Kawasaki disease-induced colitis were identified in the adult population. Colitis induced by medium-to-small vessel vasculitis was reported in 6 cases; 2 secondary to EGPA and 4 secondary to granulomatosis with polyangiitis (GPA) [19, 23, 26, 28, 29, 32]. Small-vessel vasculitis was implicated in 2 cases: 1 case of immunoglobulin A vasculitis (Henoch-Schönlein purpura) and 1 case of microscopic polyangiitis [22, 31]. Colitis induced by vasculitis associated with systemic disease was identified in 3 cases caused by systemic lupus erythematosus (SLE) [18, 21, 30]. Six cases had variable vessel vasculitis (Behçet disease) [15–17, 20, 25, 27]. All patients suffered from ischemic colitis, and 7 patients also had hemorrhagic colitis [18, 19, 21, 22, 24, 25, 30]. Twelve patients (60.0%) showed systemic signs of vasculitis, including cutaneous vasculitis (9 of 20), orogenital ulceration (4 of 20), arthralgia (3 of 20), and pulmonary eosinophilia (2 of 20) [15–17, 21, 25–28, 30–32]. Five patients (25.0%) had a vasculitis diagnosis prior to presenting with colitis [17, 18, 20, 21, 28].
All patients with SLE-induced colitis (n = 3) tested positive for ANA and double-stranded DNA (dsDNA) antibody [18, 21, 30]. All patients with GPA (n= 4) tested positive for c-ANCA [19, 23, 28, 29]. One patient with EGPA tested positive for ANA and another positive for p-ANCA [26, 32]. The patient with microscopic polyangiitis tested positive for p-ANCA [22]. Additionally, 2 patients with Behçet disease tested positive for the HLA-B51 allele and 1 for the HLA-B5 allele [25, 27].
Eighteen patients (90.0%) received CT for investigation, and 17 (85.0%) underwent a colonoscopy or sigmoidoscopy (Table 2) [14–32]. Patients received between 1 and 3 colonoscopies or sigmoidoscopies, with an average of 1.3 per patient. The endoscopy findings were described as “pancolitis,” “patchy ulceration,” “inflammatory cell infiltration,” and “hematoma [25] hemorrhage.” Eight patients received surgical intervention; 50.0% had immediate surgery on presentation, and the remaining 4 underwent surgery after failed medical management (Table 2) [14, 20, 24, 25, 29–32]. On average, the surgically treated patients underwent 1.6 procedures. All operated patients underwent an initial laparotomy, and 62.5% proceeded to stoma formation (ileostomy in 2 patients, colostomy in 2, and Hartmann with stoma formation in 1) [14, 20, 24, 25, 29–32]. Twenty-five percent underwent a right hemicolectomy [25, 29]. Medical therapy included steroids (prednisolone and methylprednisolone), monoclonal antibodies (rituximab, infliximab, and mepolizumab), disease-modifying antirheumatic drugs (DMARDs; sulfasalazine, mesalazine, azathioprine, and cyclophosphamide), and plasmapheresis, as shown in Table 3. Ninety-five percent of patients received immunosuppression (either initial or after the index operation). Steroids (70.0%) were the most common medical therapy, followed by DMARDs (60.0%), monoclonal antibodies (15.0%), and plasmaphereses (10.0%) [14–19, 21–32]. The timeframe of failed medical therapy was variable (7–66 days) [14, 20, 29, 30].
The median length of stay was 40.5 days (Table 3) [14–32]. One patient with SLE developed hospital-acquired pneumonia and gram-negative septicemia [21]. Another patient with Behçet disease developed an enterocutaneous fistula and central line sepsis following emergency laparotomy with end-ileostomy [20]. The median follow-up was 11 months [14–32]. Two patients suffered a relapse and developed colitis again [16, 29]. Only 1 patient was initially misdiagnosed with IBD but was later confirmed to have vasculitis-induced colitis (without concomitant IBD) [14]. Five patients had stoma formation, and stoma reversal was not reported [14, 20, 24, 25, 29].
This case report and literature review provides insight into the presentation, diagnosis, and medical and surgical management of vasculitis-induced colitis in the absence of IBD. Extensive literature has reported on the relationship between IBD and vasculitis [6]. However, until now, vasculitis-induced colitis in the absence of IBD has been a poorly understood entity.
Our patient was younger (20 years old) than the average age of 49 years identified in this literature review. No sex predilection was identified in the review. In keeping with the patient presented herein, all patients from the literature review had ischemic colitis, with an intermittent presentation of hemorrhagic colitis. The systemic signs of vasculitis identified in our patient were identified in 60% of patients in the literature review and should be considered in the clinical examination of patients presenting with the first episode of colitis. As demonstrated in our case report, vasculitis-induced colitis can occur in patients without a prior known history of vasculitis. This was reinforced by the literature review, wherein 75% of patients had also not received a prior vasculitis diagnosis [16, 18, 19, 31]. The eventual diagnosis and final treatment of our patient relied on serological testing (c-ANCA); likewise, in the literature review, 60% of patients required serological rheumatological testing (ANA, dsDNA, HLA-B5, HLA-B51, p-ANCA, or c-ANCA) to confirm the diagnosis. Preoperatively, the patient showed worsening inflammatory markers (increasing white cell and C-reactive protein count), alongside a declining hematology profile (decreasing hemoglobin, albumin, and platelet count) which highlighted his worsening clinical status despite immunosuppression, and guided the decision to proceed to emergency surgery. Clinically, despite immunosuppression, his rectal bleeding increased, alongside worsening abdominal and bilateral knee pain, which further highlighted that immunosuppression was insufficient for this patient. In our case, the preoperative serology and clinical condition of the patient worsened despite immunosuppression for his colitis, which ultimately led to an emergency laparotomy. Clinicians should be aware of this possibility. This study, which presents a review and case report, highlights that all patients who experience colitis without a known etiology should receive rheumatological serology in addition to a careful clinical examination to further aid their diagnostic process.
Our patient had systemic signs of vasculitis on presentation (joint pain and cystitis). This was consistent with cases reported in the literature, where 60% of patients with acute colitis have systemic signs of vasculitis, including cutaneous vasculitis, orogenital ulceration, arthralgia, and pulmonary vasculitis [15–17, 21, 25–28, 30–32]. On the contrary, only 25.0% of patients had existing vasculitis [17, 18, 20, 21, 28]. This suggests that vasculitis may have been suspected based on systemic signs, before the patient progressed to life-threatening colitis. In comparison, the development of vasculitis tends to occur well after an initial diagnosis of IBD [6]. Hence, the early finding of systemic vasculitis signs alongside new-onset colitis may offer a clue in the differential diagnosis for the clinician in addition to the more common IBD differential. Ultimately, our patient required a diagnosis based on both pathological and clinical findings. Finally, colorectal surgery was required in our case following failed medical therapy (steroids, monoclonal antibodies, DMARDs, and plasmapheresis), as was also identified in 20% of the patients in the literature [14, 20, 29, 30].
Seven patients (35.0%) showed evidence of hemorrhagic colitis on either endoscopic or surgical evaluation. Recognizing hemorrhagic colitis in cases of vasculitis-induced colitis is critical. Rapid hemorrhage, particularly into the retroperitoneum (as occurred in our case report) is easy to miss and can result in life-threatening blood loss. Hadi et al. [30] reported a case of SLE-induced colitis with intraperitoneal hemorrhage, which ultimately required 7 units of blood and extensive hemodynamic support. Hemorrhagic colitis is typically seen secondary to infection with Escherichia coli O157, associated with hemolytic uremic syndrome [33]. This review highlights that vasculitis-induced colitis can present with hemorrhagic colitis, and should be considered as a differential alongside E. coli infection. CT is useful where colitis is suspected due to its role in detecting diffuse colitis and its ability to detect abdominal complications requiring immediate surgical intervention [34]. The consideration of endoscopic investigation and its safety also warrant discussion, as bowel preparation is a risk factor for developing colitis and a complete colonoscopy increases the risk of perforation during active colitis [35]. Conversely, whilst sigmoidoscopy is safer, it is likely to miss colitis in all but the distal segments of the colon [36]. In our case report, vasculitis-induced colitis was identified in the right colon, transverse colon, left colon, and rectum with colonoscopy. An investigation using sigmoidoscopy alone could delay or miss a crucial diagnosis.
The diagnosis of vasculitis-induced colitis requires a high degree of clinical suspicion. This literature review has provided several diagnostic hints that can aid clinicians whilst awaiting pathological and serological results. These include the following: (1) existing/prior diagnosis of vasculitis, (2) evidence of hemorrhagic colitis, and (3) evidence of cutaneous/systemic signs of vasculitis. This review has shown that the serology and pathology results ultimately confirm the diagnosis of vasculitis-induced colitis in the absence of IBD, alongside a high degree of clinical suspicion. All patients underwent basic full blood alongside rheumatological work-up (Von Willebrand, factor V Leiden, prothrombin, thrombophilia screen, ANA, dsDNA, HLA-B5, HLA-B51, rheumatoid factor, p-ANCA, c-ANCA, hepatitis B, hepatitis C, HIV, and tuberculosis) which may have aided in the diagnosis, as it did in our case report. The formal diagnosis of vasculitis-induced colitis was ultimately identified histologically (endoscopic biopsy or surgical resection) with clinical correlation. Tissue examination revealed features atypical of either UC or CD—varying depths of inflammation, associated vascular inflammation and/or necrosis of the colon wall. Fortunately, only 1 patient in our review was initially misdiagnosed with IBD [14]. However, as the treatment for IBD and vasculitis is similar, we theorize that therapy may be effective even if the patient is initially misdiagnosed with IBD.
Eight patients with vasculitis-induced colitis required emergency surgery, of whom 4 were hemodynamically unstable on presentation and required immediate laparotomy. Of these 4 patients, only 1 required a second laparotomy. Comparatively, of the remaining 4 who had a laparotomy following failed medical management, 3 required a second laparotomy [14, 20, 24, 25, 29–32]. This suggests that prolonged medical management may lead to worse outcomes for patients and additional surgery. The timing of surgery for IBD has been extensively debated, and similarly we anticipate that the timing of surgery for vasculitis-induced colitis in the absence of IBD will require further research [37]. The decision of when to surgically intervene was guided by their clinical status (toxic megacolon, failed immunosuppression, declining nutritional status, or persistent rectal bleeding). The decision to proceed to laparotomy was viewed as a life-saving procedure, with the final operation (total colectomy with end ileostomy, right hemicolectomy, Hartmann or end-to-end ileocolic anastomosis) guided by the location of colitis [14, 20, 24, 25, 29–32]. As in our case report, stoma formation from emergency laparotomy occurred in 63% of patients. The choice of stoma formation (ileostomy, colectomy, or Hartmann procedure) in vasculitis-induced colitis was disease-dependent. It is known that emergency stoma formation is associated with a higher complication rate than in elective cases due to edema and inflammation of the bowel. The emergent nature of the colitis disease process precludes the ability to adequately prepare the colon, unlike elective stoma formation [38]. This can be further compounded by the deteriorating clinical status of the patient, particularly as seen in this literature review and case report following the failed medical management of colitis, which can lead to poor nutritional status, hemorrhage, ischemia, and significant analgesic requirements for patients. This in turn increases the risk of postoperative complications including ileus, stoma obstruction, skin necrosis, prolapse, and abscess development [37, 39]. Therefore, it could be suggested that when trialing medical management for vasculitis-induced colitis that the utmost attention is paid to potential perioperative status including nutritional care, preoperative stoma site marking (where possible), and appropriate analgesia.
This review is limited by the paucity of high-level evidence as all included studies were case reports, with the exception of 1 case series. Due to the rarity of vasculitis-induced colitis, we predict that it would be difficult to conduct a cohort study at any individual institution. Furthermore, previous reports varied greatly in outcome reporting, making patient comparison difficult. Finally, we were unable to comment on long-term outcomes of these patients, since only 4 studies documented follow-up beyond 1 year.
This literature review and case report documents known cases of vasculitis-induced colitis in the absence of IBD. Clinicians should be aware that vasculitis-induced colitis can exist without IBD and be successfully managed, with a combination of colorectal intervention and immunosuppression, as shown by our case of a 20-year-old male patient with vasculitis-induced colitis secondary to c-ANCA positive EGPA. We have highlighted factors that should raise clinical suspicion of this diagnosis and differentiate it from colitis associated with IBD. Future research should explore longer-term outcomes in these patients.
Fig. 1.
Computed tomography findings revealing complete pancolitis, most marked in the sigmoid colon and rectum, with no evidence of perforation or abscess. There was masked bowel wall thickening and edema involving the rectum and sigmoid colon, and to a lesser extent, the large bowel. No evidence of free gas or free fluid was found, with preserved mucosal enhancement.
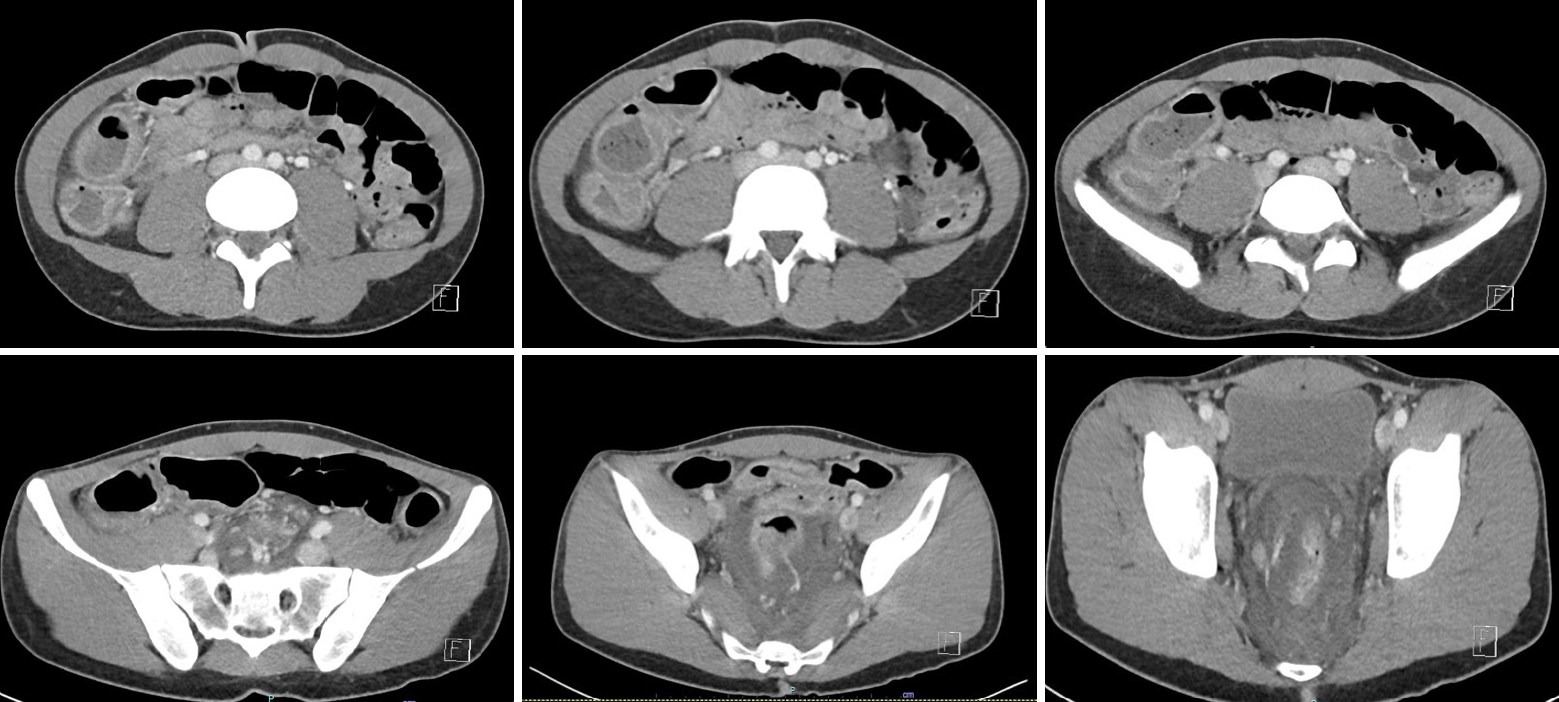
Fig. 2.
Colonoscopy findings. Severe colitis was shown in the rectum (A), sigmoid colon (B), descending colon (C), and transverse colon (D).
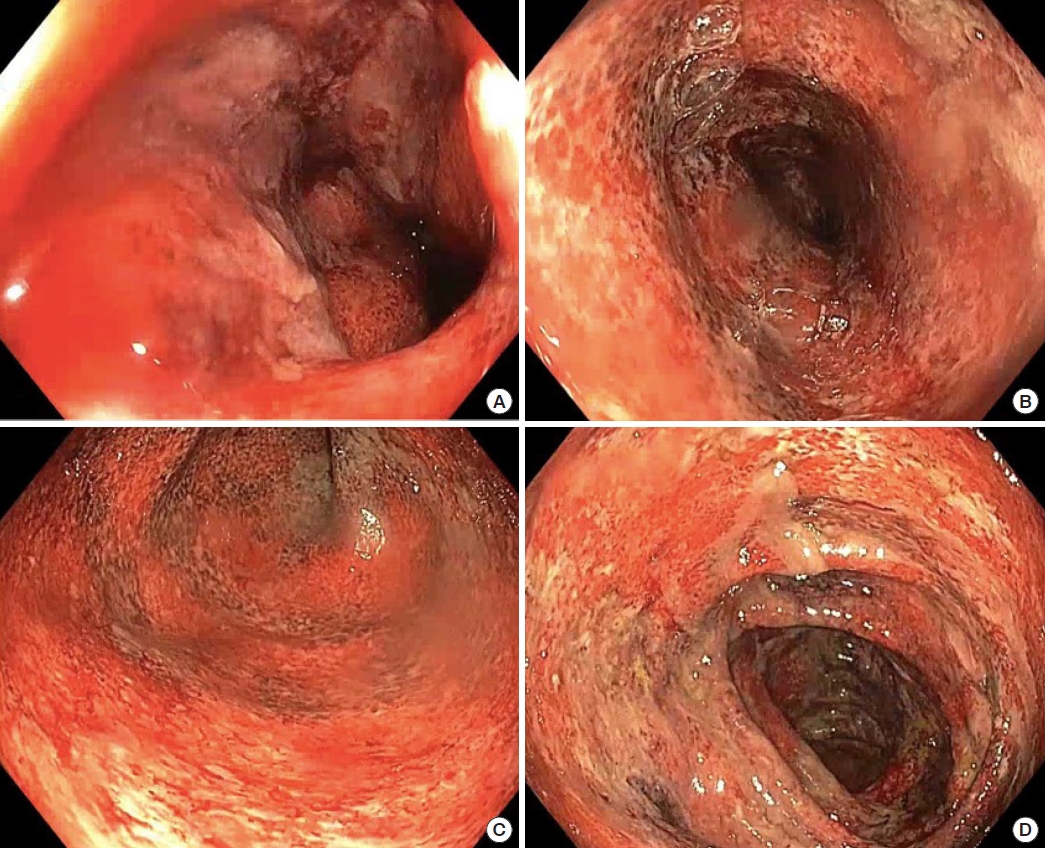
Fig. 3.
Histology (hematoxylin-eosin staining, ×400) and gross colectomy specimen findings. (A) Thrombotic vessels. (B) Mucosal inflammation with crypt abscesses. (C) Lymph node with vessel thrombosis. (D) Thrombotic vessels with mild inflammation of the vessel wall. (E) Gross colectomy specimen.
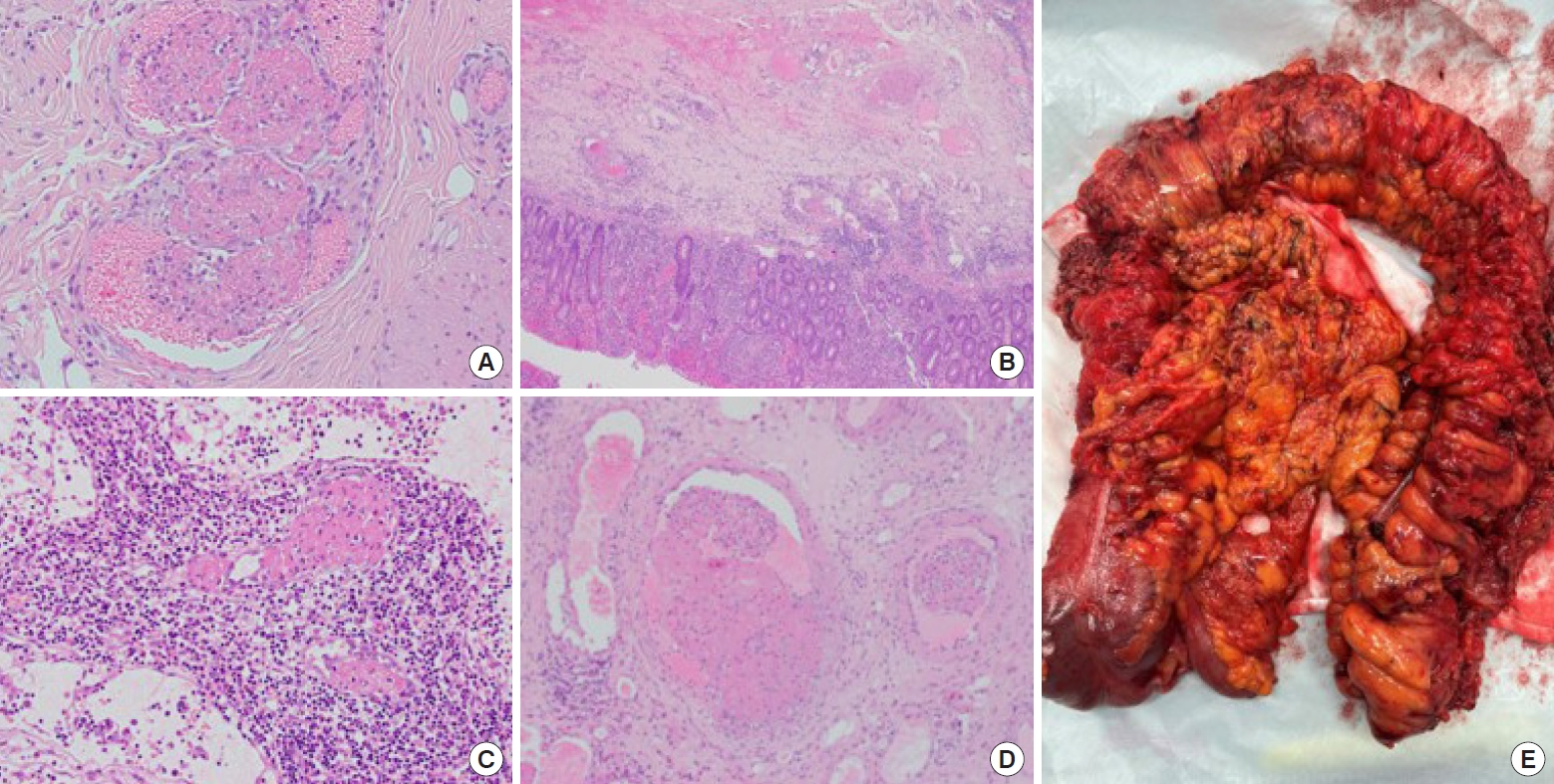
Fig. 4.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) flowchart for this literature review.
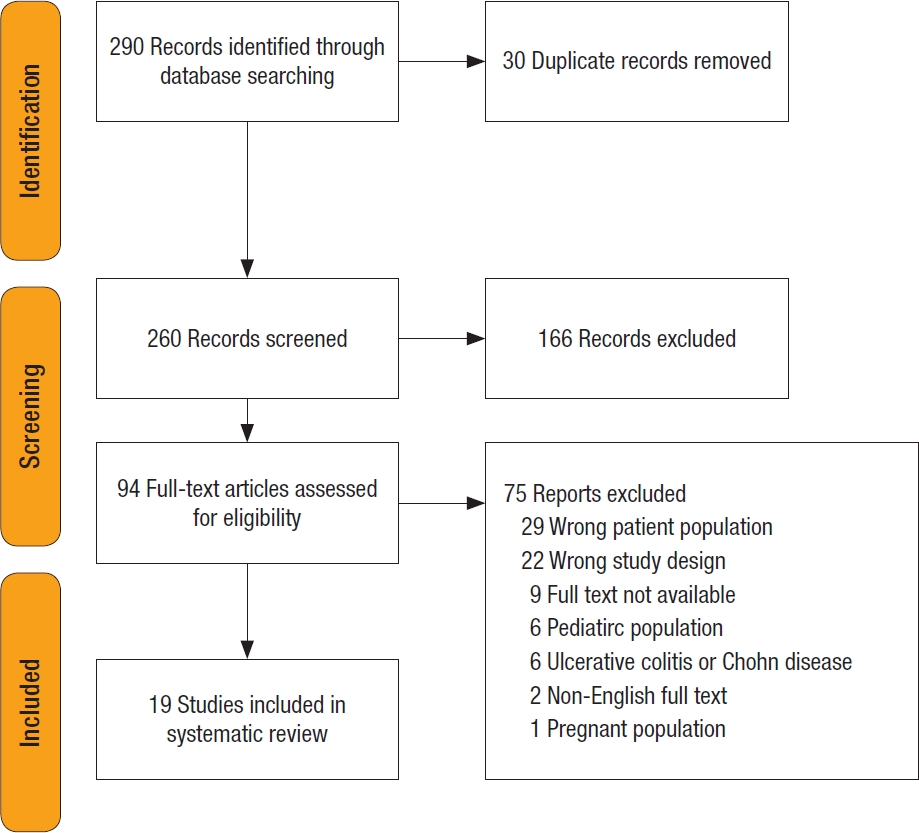
Table 1.
Vasculitis-induced colitis patient characteristics: patient demographics, vasculitis diagnosis, serological findings, and systemic findings
| Study | Study design | Age (yr) | Sex | Vasculitis | Vasculitis diagnosis prior to the colitis | Positive serologic test | Systemic vasculitis | Ischemic colitis | Hemorrhagic colitis |
|---|---|---|---|---|---|---|---|---|---|
| Okada et al. [14] (1999) | Case report | 60 | M | Polyarteritis nodosa | No | - | None | Yes | No |
| Naganuma et al. [15] (2002) | Case report | 37 | F | Behçet disease | No | - | Cutaneous vasculitis, Orogenital ulceration | Yes | No |
| Kram et al. [16] (2003) | Case report | 35 | F | Behçet disease | No | - | Cutaneous vasculitis, Orogenital ulceration | Yes | No |
| Kim et al. [17] (2007) | Case report | 39 | F | Behçet disease | Yes | - | Orogenital ulceration, cutaneous vasculitis, arthralgia | Yes | No |
| Lee et al. [18] (2008) | Case report | 32 | M | SLE | Yes | ANA, dsDNA | None | Yes | Yes |
| Qian et al. [19] (2010) | Case report | 79 | F | GPA | No | c-ANCA | None | Yes | Yes |
| Adiamah and Wong [20] (2010) | Case report | 50 | F | Behçet disease | Yes | - | None | Yes | No |
| Tayal et al. [21] (2011) | Case report | 46 | F | SLE | Yes | ANA, dsDNA | Arthralgia, alopecia, | Yes | Yes |
| Fukushima et al. [22] (2013) | Case report | 70 | F | Microscopic polyan- giitis | No | p-ANCA | None | Yes | Yes |
| Sinnott et al. [23] (2013) | Case report | 29 | M | GPA | No | c-ANCA | None | Yes | No |
| Hamzaoui et al. [24] (2013) | Case report | 55 | M | Polyarteritis nodosa | No | - | None | Yes | Yes |
| Shahverdi et al. [25] (2017) | Case report | 62 | F | Behçet disease | No | HLA-B5, HLA-B51 | Retinal vasculitis, cutaneous vasculitis | Yes | Yes |
| Ameneiros-Lago et al. [26] (2018) | Case report | 74 | F | EGPA | No | ANA | Pulmonary eosinophilia | Yes | No |
| Tominaga et al. [27] (2018) | Case report | 40 | M | Behçet disease | No | HLA-B51 | Orogenital ulceration, cutaneous vasculitis, arthralgia | Yes | No |
| Pan et al. [28] (2018) | Case report | 45 | M | GPA | Yes | c-ANCA | Sinusitis and cutaneous vasculitis | Yes | No |
| Sato et al. [29] (2019) | Case report | 55 | M | GPA | No | c-ANCA | None | Yes | No |
| Hadi et al. [30] (2020) | Case report | 39 | F | SLE | No | ANA, dsDNA | Cutaneous vasculitis | Yes | Yes |
| Catal et al. [31] (2021) | Case series | 60 | M | IgA (HSP) | No | - | Cutaneous vasculitis | Yes | No |
| 45 | M | IgA (HSP) | No | Cutaneous vasculitis | Yes | No | |||
| Vasandani et al. [32] (2022) | Case report | 28 | M | EGPA | No | p-ANCA | Pulmonary eosinophilia | Yes | No |
M, male; F, female; SLE, systemic lupus erythematosus; ANA, antinuclear antibodies; dsDNA, double-stranded DNA; GPA, granulomatosis with polyangiitis; c-ANCA, cytoplasmic antineutrophil cytoplasmic antibody; p-ANCA, perinuclear antineutrophil cytoplasmic antibody; EGPA, eosinophilic granulomatosis with polyangiitis; IgA, immunoglobulin A; HSP, Henoch-Schönlein purpura.
Table 2.
Vasculitis-induced colitis in the absence of inflammatory bowel disease according to colonoscopy, sigmoidoscopy and operative findings
| Study | Colonoscopy/sigmoidoscopy feature | Colonoscopy/sigmoidoscopy histopathology | Surgery | Surgical pathology |
|---|---|---|---|---|
| Okada et al. [14] (1999) | Perforation of the sigmoid colon with ischemic change due to polyarteritis nodosa | Loss of sigmoid colon mucosa with ulcers | 1. Laparotomy (closure of the perforation, lavage, drainage, transverse colostomy) | Thickened, contracted, stiff mesocolon and descending colon with exudate present. Perforation in the sigmoid colon. |
| 2. Hartmann procedure | ||||
| Naganuma et al. [15] (2002) | Noncaseating epithelioid granuloma | - | No surgery | - |
| Kram et al. [16] (2003) | Deep punched-out ulcerations throughout transverse, ascending colon, and ileum | - | No surgery | - |
| Kim et al. [17] (2007) | Longitudinal ulcers and inflammatory pseudopolyps | Shallow ulcerations with inflammatory infiltration consisting of lymphocytes and plasma cells | No surgery | - |
| Lee et al. [18] (2008) | Inflammatory cell infiltration, mucosal hemorrhage+, small vessel wall thicken- ing with lymphocyte infiltration | - | No surgery | - |
| Qian et al. [19] (2010) | Pancolitis with ulcers had erythematous and edematous borders with a white exudative base | Colonic mucosa evidenced foci of ulceration, inflammation, and hemorrhage within the lamina propria | No surgery | - |
| Adiamah and Wong [20] (2010) | - | Bowel ulceration | 1. Laparotomy with end ileostomy | Perforated caecum and sepsis, with abscess perforation. Transmural inflammation with a punched-out lesion. |
| 2. Fluid collection drained with pigtails | ||||
| 3. Laparotomy | ||||
| Tayal et al. [21] (2011) | Granular mucosa with contact bleeding and hemorrhage | Focal neutrophilic activity, vessel wall infiltration, lamina propria, cryptitis, and crypt abscess | No surgery | - |
| Fukushima et al. [22] (2013) | Hemorrhagic, irregular ulcers | Inflammatory cell infiltration (including lymphocytes, neu- trophils, and eosinophils), intestinal edema, and crypt destruction | No surgery | - |
| Sinnott et al. [23] (2013) | Patchy mild erythema and ulceration. | - | No surgery | - |
| Hamzaoui et al. [24] (2013) | Sigmoid colon ulcerative and bleeding | Fibrinoid necrosis and destruction of the internal lamina in small and medium-size arteries, which are rich in plasma cells, lymphocytes, and neutrophils | Laparotomy: resection and ileostomy | Ischemic, cyanosed, and violaceous colon. Fibrinoid necrosis and destruction of the internal lamina in small and medium-size arteries, which are rich in plasma cells, lymphocytes, and neutrophils. |
| Shahverdi et al. [25] (2017) | - | - | Laparotomy, right hemicolectomy, and end-to-end ileocolic anastomosis | Hemorrhagic infarction with marked neutrophilic necrotizing inflammation involving the mucosa and submucosa. Submucosal acute necrotizing inflammation and necrotizing vasculitis of medium and small vessels. |
| Ameneiros-Lago et al. [26] (2018) | Patchy erythematous areas in the left colon with abundant mucoid secretion | Eosinophilic inflammatory infiltrate of perivascular distribution | No surgery | - |
| Tominaga et al. [27] (2018) | Continual abnormal mucosal vascular pattern, friability, ulcerations, and granular changes | Mild-to-moderate infiltration of inflammatory cells | No surgery | - |
| Pan et al. [28] (2018) | Pancolitis with widespread ulceration | Inflammatory granulation tissues | No surgery | - |
| Sato et al. [29] (2019) | Punched-out ulcerative lesion in the caecum and a semicircular punched-out ulcerative lesion in the descending colon | - | 1. Laparotomy (partial resection and colectomy) on the 66th day | 1. Descending colon perforation and ischemia |
| 2. Right hemicolectomy and ileostomy on the 72th day | 2. Ascending colon necrotizing colitis | |||
| Hadi et al. [30] (2020) | Ischemic rectum with bulging tense, hematoma | - | Laparotomy, proctectomy, and colostomy, with abdominal washout | Two gross transmural perforations, ischemic proctocolitis, acute serositis, fat necrosis, lymphocytic infiltration. |
| Catal et al. [31] (2021) | - | - | 1. Laparotomy | Edema, no gangrene, widespread inflammation. |
| 2. Laparotomy | ||||
| Vasandani et al. [32] (2022) | Reactive type-lymphoid cells | - | Laparotomy | Florid serositis, hypereosinophilic cell population in the right colon. |
Table 3.
Postoperative outcomes and medical management of vasculitis-induced colitis in the absence of inflammatory bowel disease
| Study | Steroids | Monoclonal antibodya | DMARDb | Plasmaphereses | Subsequent relapse | Length of stay (day) | Latest follow-up (mo) | Misdiagnosis of UC | 30-day Morbidity | Complication |
|---|---|---|---|---|---|---|---|---|---|---|
| Okada et al. [14] (1999) | Yes | No | Yes | No | NA | NR | NR | Yes | Colostomy | - |
| Naganuma et al. [15] (2002) | Yes | No | Yes | No | No | 90 | 36 | No | - | - |
| Kram et al. [16] (2003) | Yes | No | Yes | No | Yes | NR | 8 | No | - | - |
| Kim et al. [17] (2007) | Yes | No | Yes | No | No | 60 | 12 | No | - | - |
| Lee et al. [18] (2008) | Yes | No | No | No | NA | 9 | NR | No | - | - |
| Qian et al. [19] (2010) | No | No | Yes | Yes | NA | NR | NR | No | - | - |
| Adiamah and Wong [20] (2010) | No | No | No | No | NA | 62 | NR | No | Rectovaginal fistula, ileostomy, malnourishment | Rectovaginal enterocutaneous fistula, central line sepsis |
| Tayal et al. [21] (2011) | No | Yes | No | No | No | NR | 6 | No | - | HAP and gramnegative septicemia |
| Fukushima et al. [22] (2013) | Yes | No | Yes | No | NA | NR | NR | No | - | - |
| Sinnott et al. [23] (2013) | Yes | No | Yes | Yes | No | NR | 0.33 | No | - | - |
| Hamzaoui et al. [24] (2013) | Yes | No | Yes | No | No | NR | 12 | No | Ileostomy | - |
| Shahverdi et al. [25] (2017) | No | No | No | No | No | 12 | 0.39 | No | - | - |
| Ameneiros-Lago et al. [26] (2018) | Yes | No | Yes | No | No | NR | 3.7 | No | - | - |
| Tominaga et al. [27] (2018) | Yes | No | Yes | No | No | NR | 48 | No | - | - |
| Pan et al. [28] (2018) | Yes | No | Yes | No | NA | 10 | NR | No | - | - |
| Sato et al. [29] (2019) | No | Yes | Yes | No | Yes | 103 | 3 | No | Ileostomy | - |
| Hadi et al. [30] (2020) | Yes | No | No | No | No | 21 | 6 | No | Colostomy, lupus nephritis | - |
| Catal et al. [31] (2021) | Yes | No | No | No | No | NR | 6 | No | - | - |
| Yes | No | No | No | No | NR | 6 | No | - | - | |
| Vasandani et al. [32] (2022) | No | Yes | No | No | No | NR | 4 | No | - | - |
REFERENCES
1. Camilleri M, Pusey CD, Chadwick VS, Rees AJ. Gastrointestinal manifestations of systemic vasculitis. Q J Med 1983;52:141–9.

2. Conrad K, Roggenbuck D, Laass MW. Diagnosis and classification of ulcerative colitis. Autoimmun Rev 2014;13:463–6.


3. Laass MW, Roggenbuck D, Conrad K. Diagnosis and classification of Crohn’s disease. Autoimmun Rev 2014;13:467–71.


4. Ausch C, Madoff RD, Gnant M, Rosen HR, Garcia-Aguilar J, Hölbling N, et al. Aetiology and surgical management of toxic megacolon. Colorectal Dis 2006;8:195–201.


5. Wilson JC, Furlano RI, Jick SS, Meier CR. Inflammatory bowel disease and the risk of autoimmune diseases. J Crohns Colitis 2016;10:186–93.


6. Sy A, Khalidi N, Dehghan N, Barra L, Carette S, Cuthbertson D, et al. Vasculitis in patients with inflammatory bowel diseases: a study of 32 patients and systematic review of the literature. Semin Arthritis Rheum 2016;45:475–82.



7. Katsanos KH, Voulgari PV, Tsianos EV. Inflammatory bowel disease and lupus: a systematic review of the literature. J Crohns Colitis 2012;6:735–42.


8. Gudbjörnsson B, Hällgren R. Cutaneous polyarteritis nodosa associated with Crohn’s disease: report and review of the literature. J Rheumatol 1990;17:386–90.

9. Bekele DI, Warrington KJ, Koster MJ. Giant cell arteritis associated with inflammatory bowel disease: a case-series and review of the literature. Rheumatol Int 2021;41:487–92.



10. International Team for the Revision of the International Criteria for Behçet’s Disease (ITR-ICBD). The International Criteria for Behçet’s Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol 2014;28:338–47.



11. Roozendaal C, Kallenberg CG. Are anti-neutrophil cytoplasmic antibodies (ANCA) clinically useful in inflammatory bowel disease (IBD)? Clin Exp Immunol 1999;116:206–13.




12. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis: a randomized study. N Engl J Med 1987;317:1625–9.


13. Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65:1–11.


14. Okada M, Konishi F, Sakuma K, Kanazawa K, Koiwai H, Kaizaki Y. Perforation of the sigmoid colon with ischemic change due to polyarteritis nodosa. J Gastroenterol 1999;34:400–4.



15. Naganuma M, Iwao Y, Kashiwagi K, Funakoshi S, Ishii H, Hibi T. A case of Behçet’s disease accompanied by colitis with longitudinal ulcers and granuloma. J Gastroenterol Hepatol 2002;17:105–8.


16. Kram MT, May LD, Goodman S, Molinas S. Behçet’s ileocolitis: successful treatment with tumor necrosis factor-alpha antibody (infliximab) therapy: report of a case. Dis Colon Rectum 2003;46:118–21.

17. Kim ES, Chung WC, Lee KM, Lee BI, Choi H, Han SW, et al. A case of intestinal Behcet’s disease similar to Crohn’s colitis. J Korean Med Sci 2007;22:918–22.



18. Lee JR, Paik CN, Kim JD, Chung WC, Lee KM, Yang JM. Ischemic colitis associated with intestinal vasculitis: histological proof in systemic lupus erythematosus. World J Gastroenterol 2008;14:3591–3.



19. Qian Q, Cornell L, Chandan V, Hartman R, Caples S. Hemorrhagic colitis as a presenting feature of Wegener granulomatosis. J Gastrointestin Liver Dis 2010;19:445–7.

20. Adiamah A, Wong LS. Beçhet’s disease: a rare cause of rectovaginal fistula. BMJ Case Rep 2010;2010:bcr0620103130.


21. Tayal V, Chiu YH, George E, Mane S. Colitis associated with active systemic lupus erythematosus successfully treated with rituximab. J Clin Rheumatol 2011;17:79–82.


22. Fukushima M, Inoue S, Ono Y, Tamaki Y, Yoshimura H, Imai Y, et al. Microscopic polyangiitis complicated with ileal involvement detected by double-balloon endoscopy: a case report. BMC Gastroenterol 2013;13:42.




23. Sinnott JD, Matthews P, Fletcher S. Colitis: an unusual presentation of Wegener’s granulomatosis. BMJ Case Rep 2013;2013:bcr 2012007566.



24. Hamzaoui A, Litaiem N, Smiti Khanfir M, Ayadi S, Nfoussi H, Houman MH. Ischemic colitis revealing polyarteritis nodosa. Case Rep Med 2013;2013:741047.




25. Shahverdi E, Morshedi M, Oraei-Abbasian F, Allahverdi Khani M, Khodayarnejad R. A rare case of vasculitis patched necrosis of cecum due to Behçet’s disease. Case Rep Surg 2017;2017:1693737.




26. Ameneiros-Lago E, Pérez-Valcarcel J, Fernández-Fernández FJ, Caínzos-Romero T. Colitis as a form of presentation of eosinophilic granulomatosis with polyangiitis. Gastroenterol Hepatol 2018;41:302–4.


27. Tominaga K, Kamimura K, Takahashi K, Yokoyama J, Terai S. A case of Behçet’s disease with various gastrointestinal findings. Clin J Gastroenterol 2018;11:354–8.



28. Pan SW, Wang C, Zhang X, Zhang L, Yan QQ, Zhao CJ, et al. A rare endoscopic appearance of granulomatosis with polyangiitis involving the intestine: a case report. BMC Gastroenterol 2018;18:154.




29. Sato H, Shima K, Sakata H, Ohtoh T. Granulomatosis with polyangiitis with intestinal involvement successfully treated with rituximab and surgery. BMJ Case Rep 2019;12:e230355.



30. Hadi YB, Lindsay J 4th, Naqvi SF, Al-Jaroushi H. Systemic lupus erythematosus presenting with ischemic proctitis and abdominal compartment syndrome. Case Rep Gastrointest Med 2020;2020:5723403.




31. Catal O, Ozer B, Sit M. Henoch-Schönlein purpura presenting with acute abdomen. J Coll Physicians Surg Pak 2021;31:350–2.


32. Vasandani N, Isaac M, Bajwa A, Sheehan M, Nugent E. A surgical presentation of Churg-Strauss syndrome. Cureus 2022;14:e24342.



33. Nayar DM, Vetrivel S, McElroy J, Pai P, Koerner RJ. Toxic megacolon complicating Escherichia coli O157 infection. J Infect 2006;52:e103–6.


34. Imbriaco M, Balthazar EJ. Toxic megacolon: role of CT in evaluation and detection of complications. Clin Imaging 2001;25:349–54.


35. Makkar R, Bo S. Colonoscopic perforation in inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2013;9:573–83.


36. Tanaka M, Mazzoleni G, Riddell RH. Distribution of collagenous colitis: utility of flexible sigmoidoscopy. Gut 1992;33:65–70.



37. Roberts SE, Williams JG, Yeates D, Goldacre MJ. Mortality in patients with and without colectomy admitted to hospital for ulcerative colitis and Crohn’s disease: record linkage studies. BMJ 2007;335:1033.



- TOOLS








