- Search
| Ann Coloproctol > Epub ahead of print |
|
Abstract
Purpose
Methods
Results
Notes
Acknowledgments
The corresponding author would like to thank the team for the expertise and assistance in all aspects of the study and for the help in writing the manuscript, in particular Professor G. B. Cadiere for the correction of the draft and his advice.
Author contributions
Conceptualization:
Data curation:
Formal analysis:
Investigation:
Methodology:
Project administration:
Resources:
Software:
Supervision:
Validation:
Visualization:
WritingŌĆōoriginal draft:
WritingŌĆōreview & editing: all authors.
All authors read and approved the final manuscript.
Additional information
This study was previously shared as a preprint on Research Square (https://doi.org/10.21203/rs.3.rs-1392300/v2) and has since undergone peer review for publication in this journal.
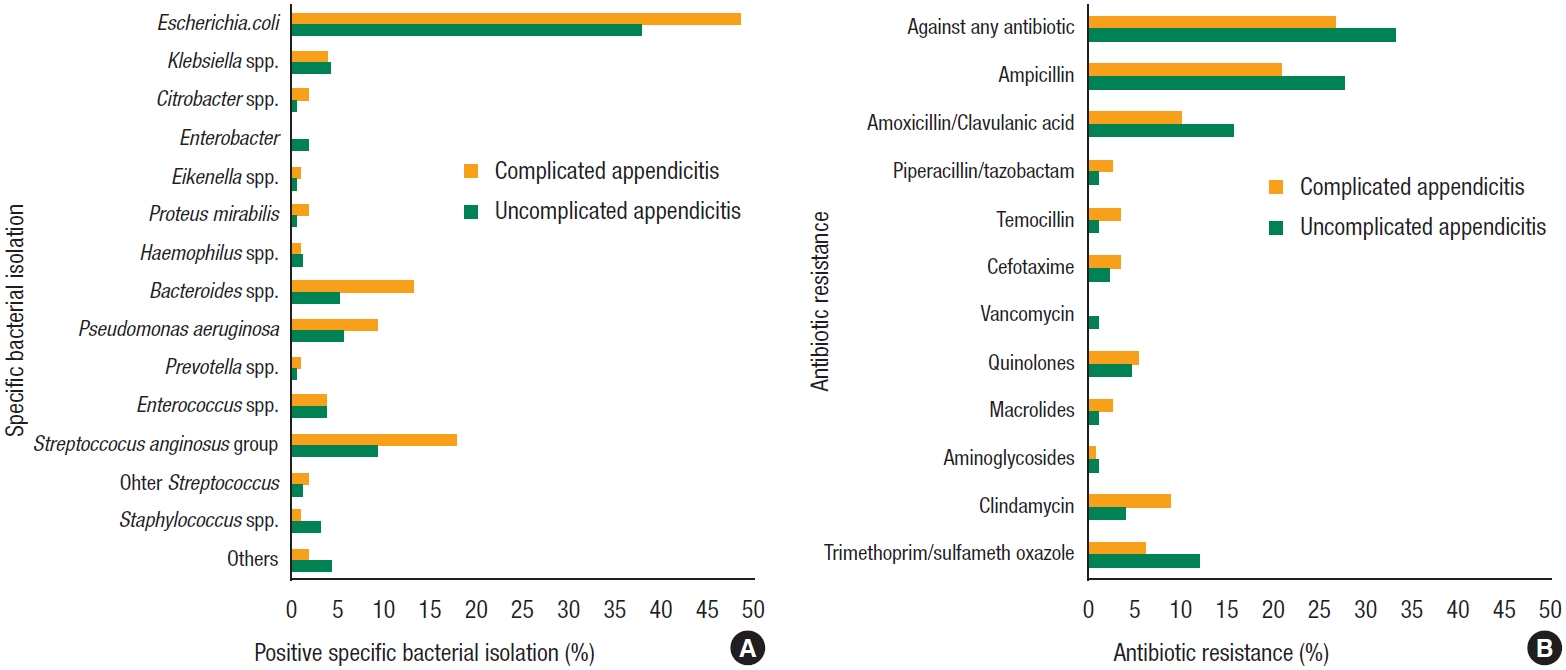
Fig.┬Ā1.
Table┬Ā1.
| Characteristic | Acute appendicitis | P-value | |
|---|---|---|---|
| Uncomplicated (n=161) | Complicated (n=107) | ||
| Age (yr) | 33.5 (25.3ŌĆō41.0) | 38.5 (27.0ŌĆō52.0) | 0.018* |
| Sex | 0.016* | ||
| ŌĆāFemale | 70 (43.5) | 31 (29.0) | |
| ŌĆāMale | 91 (56.5) | 76 (71.0) | |
| Nationality | 0.250 | ||
| ŌĆāEuropean | |||
| ŌĆāŌĆāBelgian | 59 (36.6) | 44 (41.1) | |
| ŌĆāŌĆāNon-Belgian | 42 (26.1) | 35 (32.7) | |
| ŌĆāAfrican | 34 (21.1) | 20 (18.7) | |
| ŌĆāMiddle Eastern | 7 (4.3) | 2 (1.9) | |
| ŌĆāAsian | 5 (3.1) | 1 (0.9) | |
| ŌĆāAmerican | 9 (5.6) | 1 (0.9) | |
| ŌĆāUnknown | 5 (3.1) | 4 (3.7) | |
| Charlson Comorbidity Index | 0 (0ŌĆō0) | 0 (0ŌĆō1) | 0.017* |
| History of abdominal surgery | 26 (16.1) | 14 (13.1) | 0.490 |
| History of other surgeries | 18 (11.2) | 10 (9.3) | 0.631 |
| Physical examination at admission | |||
| ŌĆāHeart rate (bpm) | 87 (76.0ŌĆō100.5) | 95 (81.0ŌĆō108.0) | 0.007* |
| ŌĆāTachycardia (>100 bpm) | 48 (29.8) | 46 (43.0) | 0.027* |
| ŌĆāSystolic arterial pressure (mmHg) | 125 (118ŌĆō138) | 130 (118ŌĆō140) | 0.286 |
| ŌĆāHypotension (<100 mmHg) | 8 (5.0) | 7 (6.5) | 0.583 |
| ŌĆāTemperature (┬░C) | 36.9 (36.5ŌĆō37.4) | 37.0 (36.7ŌĆō37.5) | 0.039* |
| ŌĆāFever (>37.8 ┬░C) | 19 (11.8) | 18 (16.8) | 0.243 |
| ŌĆāSeptic shock | 4 (2.5) | 2 (1.9) | >0.999 |
| Blood test at admission | |||
| ŌĆāC-reactive protein (g/dL) | 29.2 (6.5ŌĆō101.0) | 97.5 (29.6ŌĆō188.3) | <0.001* |
| ŌĆāWhite blood cell count (/mm3) | 13,460 (8,216ŌĆō16,170) | 15,720 (12,040ŌĆō18,500) | 0.001* |
| Surgical intervention | 0.020* | ||
| ŌĆāAppendicectomy | 158 (98.1) | 99 (92.5) | |
| ŌĆāCecectomy | 1 (0.6) | 7 (6.5) | |
| ŌĆāIleocecal resection | 2 (1.2) | 1 (0.9) | |
| Laparoscopic procedure | 156 (96.9) | 91 (85.0) | <0.001* |
| Drainage | 83 (51.6) | 84 (78.5) | <0.001* |
| ŌĆāPeriappendicular | 42 (26.1) | 59 (55.1) | <0.001* |
| ŌĆāPelvic | 63 (39.1) | 57 (53.3) | 0.025* |
| ŌĆāOther localizations | 6 (3.7) | 4 (3.7) | >0.999 |
Table┬Ā2.
| Variable | Acute appendicitis | P-value | |
|---|---|---|---|
| Uncomplicated (n=161) | Complicated (n=107) | ||
| Culture | 0.016* | ||
| ŌĆāNegative | 75 (46.6) | 34 (31.8) | |
| ŌĆāPositive | 86 (53.4) | 73 (68.2) | |
| Bacterial isolation | |||
| ŌĆāGram-negative | 76 (47.2) | 65 (60.7) | 0.030* |
| ŌĆāGram-positive | 26 (16.1) | 24 (22.4) | 0.196 |
| ŌĆāStrict anaerobes | 18 (11.2) | 22 (20.6) | 0.035* |
| First-line antibiotic treatment | 0.013* | ||
| ŌĆāNone | 7 (4.3) | 2 (1.9) | |
| ŌĆāMonotherapy | 154 (95.7) | 100 (93.5) | |
| ŌĆāCombined therapy | 0 (0) | 5 (4.7) | |
| First-line antibiotic treatment | |||
| ŌĆāNone | 7 (4.3) | 2 (1.9) | 0.098 |
| ŌĆāAmoxicillin/clavulanic acid | 152 (94.4) | 96 (89.7) | |
| ŌĆāAmoxicillin/clavulanic acid-metronidazole | 0 (0) | 1 (0.9) | |
| ŌĆāAmoxicillin/clavulanic acid-ornidazole | 0 (0) | 1 (0.9) | |
| ŌĆāCefuroxime-metronidazole | 0 (0) | 1 (0.9) | |
| ŌĆāCefuroxime-ornidazole | 0 (0) | 2 (1.9) | |
| ŌĆāClindamycin | 2 (1.2) | 1 (0.9) | |
| ŌĆāLevofloxacin | 0 (0) | 1 (0.9) | |
| ŌĆāPiperacillin/tazobactam | 0 (0) | 2 (1.9) | |
| Resistance to first-line antibiotic treatment | 27 (16.8) | 10 (9.3) | 0.084 |
| Second-line antibiotic treatment | 0.889 | ||
| ŌĆāNone | 132 (80.2) | 86 (80.4) | |
| ŌĆāMonotherapy | 11 (6.8) | 9 (8.4) | |
| ŌĆāCombined therapy | 18 (11.2) | 12 (11.2) | |
| Second-line antibiotic treatment | |||
| ŌĆāNone | 132 (82.0) | 86 (80.4) | 0.763 |
| ŌĆāAmoxicillin/clavulanic acid-metronidazole | 0 (0) | 1 (0.9) | |
| ŌĆāAmoxicillin/clavulanic acid-ornidazole | 6 (3.7) | 4 (3.7) | |
| ŌĆāAmoxicillin/clavulanic acid-doxycycline | 1 (0.6) | 0 (0) | |
| ŌĆāCefuroxime-metronidazole | 1 (0.6) | 0 (0) | |
| ŌĆāCefuroxime-ornidazole | 1 (0.6) | 0 (0) | |
| ŌĆāCeftriaxone-doxycycline | 2 (1.2) | 0 (0) | |
| ŌĆāDoxycycline | 0 (0) | 1 (0.9) | |
| ŌĆāLevofloxacin | 3 (1.9) | 2 (1.9) | |
| ŌĆāLevofloxacin-ornidazole | 4 (2.5) | 4 (3.7) | |
| ŌĆāCiprofloxacin-ornidazole | 3 (1.9) | 3 (2.8) | |
| ŌĆāPiperacillin/tazobactam | 8 (5.0) | 5 (4.7) | |
| ŌĆāOrnidazole | 0 (0) | 1 (0.9) | |
| Resistance to second-line antibiotic treatment | 0 (0) | 1 (0.9) | 0.399 |
| Third-line antibiotic treatment | 0.337 | ||
| ŌĆāNone | 159 (98.8) | 103 (96.3) | |
| ŌĆāMonotherapy | 1 (0.6) | 1 (0.9) | |
| ŌĆāCombined therapy | 1 (0.6) | 1 (0.9) | |
| Third-line antibiotic treatment | 0.552 | ||
| ŌĆāNone | 159 (98.8) | 103 (96.3) | |
| ŌĆāLevofloxacin-ornidazole | 1 (0.6) | 1 (0.9) | |
| ŌĆāCiprofloxacin-ornidazole | 0 (0) | 1 (0.9) | |
| ŌĆāPiperacillin/tazobactam | 1 (0.6) | 1 (0.9) | |
| ŌĆāCeftazidime-vancomycin-ornidazole-anidulafungin | 0 (0) | 1 (0.9) | |
| Resistance to third-line antibiotic treatment | 0 (0) | 0 (0) | >0.999 |
| Antibiotic treatment duration (day) | 5.0 (2ŌĆō7) | 5.5 (3ŌĆō7) | 0.002* |
| Antibiotic treatment >1 wk | 19 (11.8) | 22 (20.6) | 0.051 |
Table┬Ā3.
| Variable | Acute appendicitis | P-value | |
|---|---|---|---|
| Uncomplicated (n = 161) | Complicated (n = 107) | ||
| Morbidity | 11 (6.8) | 21 (19.6) | 0.002* |
| ŌĆāClavien-Dindo classification of 30-day complications | 0.012* | ||
| ŌĆāŌĆāGrade I | 2 (1.2) | 6 (5.6) | |
| ŌĆāŌĆāGrade II | 4 (2.5) | 9 (8.4) | |
| ŌĆāŌĆāGrade III | 5 (3.1) | 6 (5.6) | |
| Reintervention | 3 (1.9) | 4 (3.7) | 0.442 |
| ŌĆāSpecific complication | |||
| ŌĆāŌĆāPostoperative ileus | 4 (2.5) | 6 (5.6) | 0.204 |
| ŌĆāŌĆāAcute kidney injury | 0 (0) | 1 (0.9) | 0.399 |
| ŌĆāŌĆāBacteremia | 0 (0) | 1 (0.9) | 0.399 |
| ŌĆāŌĆāSurgical site infection | 8 (5.0) | 14 (13.1) | 0.018* |
| ŌĆāŌĆāCecal perforation | 1 (0.6) | 1 (0.9) | >0.999 |
| ŌĆāBlood test at 48 hr | |||
| ŌĆāŌĆāC-reactive protein (mg/dL)┬Ā | 111.9 (28.9ŌĆō215.7) | 202.4 (96.6ŌĆō316.0) | 0.001* |
| ŌĆāŌĆāWhite blood cell count (/mm3) | 8,660 (6,715ŌĆō10,980) | 8,695 (6,335ŌĆō12,052) | 0.447 |
| ŌĆāHospital stay (day) | 3 (2ŌĆō5) | 5 (3ŌĆō6) | <0.001* |
Table┬Ā4.
| Risk factor | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age (yr) | 1.022 (1.005ŌĆō1.039) | 0.009* | 1.022 (1.005ŌĆō1.039) | 0.009* |
| Sex | 0.017* | - | - | |
| ŌĆāFemale | Reference | |||
| ŌĆāMale | 1.887 (1.120ŌĆō3.174) | |||
| Nationality | 0.249 | - | - | |
| ŌĆāEuropean (Belgian) | Reference | |||
| ŌĆāEuropean (non-Belgian) | 1.117 (0.617ŌĆō2.025) | |||
| ŌĆāAfrican | 0.789 (0.401ŌĆō1.551) | |||
| ŌĆāMiddle Eastern | 0.383 (0.076ŌĆō1.934) | |||
| ŌĆāAsian | 0.268 (0.030ŌĆō2.378) | |||
| ŌĆāAmerican | 0.149 (0.018ŌĆō1.120) | |||
| Charlson Comorbidity Index | 1.459 (1.114ŌĆō1.911) | 0.006* | 1.333 (1.003ŌĆō1.771) | 0.048* |
| History of abdominal surgery | 0.782 (0.782ŌĆō1.576) | 0.491 | - | - |
| History of other surgeries | 0.819 (0.363ŌĆō1.850) | 0.631 | - | - |
| Physical examination at admission | - | - | ||
| ŌĆāHeart rate (bpm) | 1.018 (1.004ŌĆō1.033) | 0.010* | ||
| ŌĆāTachycardia (>100 bpm) | 1.775 (1.066ŌĆō2.958) | 0.028* | ||
| ŌĆāSystolic arterial pressure (mmHg) | 1.005 (0.990ŌĆō1.019) | 0.54 | ||
| ŌĆāHypotension (<100 mmHg) | 1.339 (0.471ŌĆō3.807) | 0.584 | ||
| ŌĆāTemperature (┬░C) | 1.383 (0.994ŌĆō1.924) | 0.054 | ||
| ŌĆāFever (>37.8 ┬░C) | 1.512 (0.753ŌĆō3.035) | 0.245 | ||
| ŌĆāSeptic shock | 0.748 (0.135ŌĆō4.155) | 0.740 | ||
| Blood test at admission | ||||
| ŌĆāC-reactive protein (g/dL┬Ā) | 1.005 (1.002ŌĆō1.007) | <0.001* | - | - |
| ŌĆāWhite blood cell count (/mm3) | 1.00008 (1.00003ŌĆō1.00014) | <0.001* | 1.00007 (1.00001ŌĆō1.00013) | 0.027* |
| Surgical intervention | - | - | ||
| ŌĆāAppendicectomy | Reference | |||
| ŌĆāCecectomy | 11.172 (1.354ŌĆō92.174) | 0.025* | ||
| ŌĆāIleocecal resection | 0.798 (0.071ŌĆō8.916) | 0.855 | ||
| Laparoscopic procedure | 0.182 (0.065ŌĆō0.514) | 0.001* | 0.218 (0.073ŌĆō0.648) | 0.006* |
| Drainage | 3.432 (1.970ŌĆō5.980) | <0.001* | 2.571 (1.421ŌĆō4.653) | 0.002* |
| Culture | 0.016* | - | - | |
| ŌĆāNegative | Reference | |||
| ŌĆāPositive | 1.872 (1.123ŌĆō3.122) | |||
| Bacterial isolation | - | - | ||
| ŌĆāGram-negative | 1.731 (1.054ŌĆō2.843) | 0.030* | ||
| ŌĆāGram-positive | 1.501 (0.809ŌĆō2.787) | 0.198 | ||
| ŌĆāStrict anaerobes | 2.056 (1.043ŌĆō4.052) | 0.037* | ||
| First-line antibiotic treatment | 0.870 (0.704ŌĆō1.075) | 0.197 | - | - |
| Resistance to first-line antibiotic treatment | 0.512 (0.237ŌĆō1.106) | 0.089 | - | - |
| Bacterial specific isolation | - | - | ||
| ŌĆāEscherichia coli | 1.550 (0.945ŌĆō2.543) | 0.083 | ||
| ŌĆāKlebsiella spp. | 0.854 (0.244ŌĆō2.993) | 0.806 | ||
| ŌĆāBacteroides spp. | 2.879 (1.163ŌĆō7.124) | 0.022* | ||
| ŌĆāPseudomonas aeruginosa | 1.741 (0.683ŌĆō4.439) | 0.246 | ||
| ŌĆāEnterococcus spp. | 1.003 (0.276ŌĆō3.643) | 0.996 | ||
| ŌĆāStreptoccocus anginosus group | 2.102 (1.016ŌĆō4.347) | 0.045* | ||
REFERENCES
- TOOLS
-
METRICS

-
- 2 Crossref
- Scopus
- 1,690 View
- 68 Download
- Related articles in ACP
-
Routine Intraoperative Bacterial Culture May Be Needed in Complicated Appendicitis2020 June;36(3)
Diagnostic Value of C-reactive Protein in Complicated Appendicitis2011 June;27(3)







