Effects of angiotensin peptides on colonic motility in rats
Article information
Abstract
Purpose
Renin-angiotensin system (RAS) is involved in the pathophysiology of colonic inflammation. The aim of this study was to investigate whether small angiotensins (Angs) peptides play a role in the regulation of colonic motility and their roles are modulated in colitis.
Methods
Experimental colitis was induced by an intake of 5% dextran sulfate sodium (DSS) dissolved in tap water for 7 days in Sprague-Dawley rats. After sacrifice, plasma hormone concentrations and messenger RNAs (mRNAs) for RAS were measured. Functional analysis of colonic motility in response to Angs peptides was performed using Taenia coli.
Results
DSS-treated colon showed an increased necrosis with massive infiltration of inflammatory cells. The mRNA level of colonic angiotensin II receptor type 2 (AT2R) in DSS-treated rats was higher than that in control rats whereas the mRNA levels of angiotensin II converting enzyme (ACE), ACE2, AT1R, AT4R, and Mars receptor were not different from those in control rats. Ang III, Ang IV, and Ang-(1-9) (1, 3 μM) increased the frequency of basal colonic motility. Ang-(1-7) did not cause any significant changes in frequency and amplitude of basal motility. The order of potency for an increased frequency of basal motility seems to be Ang II>>Ang IV>Ang III=Ang-(1-9). The increased frequency of basal motility by Ang-(1-9) but not Ang IV was significantly enhanced in DSS-treated rat colon.
Conclusion
In conclusion, these data suggest that small Angs peptides are partly involved in the pathophysiological regulation of colonic motility in experimental colitis.
INTRODUCTION
Angiotensin (Ang) II is the major effective peptide of the reninangiotensin system (RAS) and play an important role in the regulation of blood pressure and fluid electrolytes balances [1]. Ang II is degraded by aminopeptidase A to Ang III, which is then degraded by aminopeptidase N to Ang IV [2–4]. In addition, Ang I and Ang II are converted to Ang-(1-9) and Ang-(1-7) by angiotensin II converting enzyme (ACE) 2, respectively, which are considered as a counter-balancing axis of RAS [5]. Several studies have shown that these small Angs peptides have different effects on cellular proliferation, hemodynamics, and hormone secretions via their own receptors. Ang II via angiotensin II receptor type 1 (AT1R) mediates cellular hypertrophy, vasoconstriction, and increases in water intake, aldosterone and vasopressin secretions whereas Ang-(1-9) [6] via angiotensin II receptor type 2 (AT2R) and Ang-(1-7) [7] via Mas receptor (MasR) mediate antihypertrophy, antiproliferation, antifibrosis, and vasodilation.
In the animal and human intestine, all the RAS components have been identified [8–10] and the presence of a local intestinal RAS has been suggested to play a physiological role in the regulation of intestinal motility and epithelial transport processes [10, 11]. In addition, RAS may be involved in the pathophysiology of inflammatory bowel disease (IBD) such as ulcerative colitis and Crohn disease [12–14]. An increased Ang II level in colonic mucosa has been reported in patients suffered from Crohn disease [15] and experimental colitis [13], and the degree of colitis is attenuated by ACE inhibitor [14, 16] and in angiotensinogen genedeficient mice [17]. However, there are a few reports on whether small Angs peptides regulate intestinal motility. We have previously reported the presence of natriuretic peptide system, an antagonistic hormone system to RAS, and their inhibitory functions on basal motility and modulation in experimental colitis [18, 19]. Therefore, the purpose of the present study is to evaluate the effects of Angs peptides on colonic motility and modulation of their responsiveness in experimental colitis.
METHODS
Ethics statements
This study was approved by the Institutional Animal Care and Use Committee of Jeonbuk National University (No. JBNU 2021-018). All of experimental protocols of this study conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication no. 85-23, revised 1996).
Animals
Male Sprague-Dawley (SD) rats, obtained from Orientbio Inc, were housed in a temperature-controlled room with a 12:12-hour light-dark cycle. The animals were provided free access to standard laboratory chow (5L79 Purina rat and mouse 18% chow, Charles River Laboratories Inc.) and water.
Dextran sulfate sodium-induced colitis
Male SD rats, weighing 200 to 220 g, were divided into 2 groups: control and experimental groups. Experimental rats received 5% dextran sulfate sodium (DSS) dissolved in tap water for 7 days and control rats received tap water [19]. On the 7th day, body weight was measured before sacrifice, and blood was collected at 4°C into prechilled tubes containing ethylenediamine-tetraacetic acid and hematocrit capillary tubes. Whole colon was removed and washed several times into 0.9% saline. Colonic weight and length were measured and were stored for the morphological analysis and messenger RNA (mRNA) measurement.
Functional analysis of colonic motility
Functional roles of Ang II, Ang III, Ang IV, Ang-(1-9), and Ang-(1-7) in the regulation of colonic motility were measured by the method previously described [18, 19]. The colonic tissue removed from rats was immediately placed in hydroxyethyl piperazine ethane sulfonicacid (HEPES) buffer (HEPES, 10 mM; NaCl, 118 mM; KCl, 4.7 mM ; CaCl2, 2.5 mM; MgSO4, 1.2 mM; NaHCO3, 25 mM; and glucose, 10 mM) saturated with O2. The colonic segment was opened along the mesenteric border and the fecal material was washed with HEPES buffer. The resulting sheet of colon was pinned out in a dissecting dish and the Taenia coli of about 3×10 mm were cut, which was mounted vertically in the organ bath by securing one end to a fixed point and the other to an isometric force transducer. The bath contained HEPES buffer gassed continuously with O2 and maintained at 37°C. Each muscle strip was equilibrated at a resting tension of 300 mg for at least 1 hour before the start of experiments. After the basal contractility was measured, the muscle strip was treated with different concentrations of Ang II, Ang III, Ang IV, Ang-(1-9), and Ang-(1-7) (0.1–10 µM) for 5 minutes and was washed twice.
Histological analysis: hematoxylin-eosin staining
Paraffinized colon tissue was deparaffinized and hydrated to water. Tissue was incubated with hematoxylin for 15 minutes, washed in running tap water for 20 minutes, and counterstained with eosin [18]. Tissue samples were dehydrated in 95% and absolute ethanol, 2 changes of 2 minutes each or until excess eosin is removed. It was cleared in xylene, 2 changes of 2 minutes each, mounted on slide for observation. The hematoxylin-eosin (H&E) stained slides were scored based on the severity of inflammation, crypt damage, and ulceration. The image of colon was taken with the digital camera (Nikon DS-U1, Nikon) and its damage was analyzed by using image analysis software (Analysis pro ver. 3.2, Soft Imaging System GmbH).
Measurement of plasma ANP concentration and renin concentration
Plasma atrial natriuretic peptide (ANP) was extracted using a Sep-Pak C18 cartridge (Waters Associates) [20] and the concentration of immunoreactive ANP in plasma was measured with a specific radioimmunoassay (RIA) [21]. Plasma renin concentration (PRC) was measured by a specific RIA of Ang I, as described previously [22].
Real-time polymerase chain reaction
Total RNA was extracted from rat colon tissue using TRIzol reagent (Invitrogen), and reverse transcription was performed using SYBR GreenER Two-Step qRT-PCR kit (Takara) [18]. The real-time (RT) polymerase chain reaction (PCR) reaction contained 10 ng of reverse-transcribed total RNA, 200 nM of forward and reverse primers, and 2× PCR master mixture in a final volume of 10 μL. PCR reaction was carried out in 384-well plates using the ABI Prism 7900HT Sequence Detection System (Applied Biosystems). All reactions were done in triplicate. Specific primers were designed using primer express software (Applied Biosystems) and their primer sequences were the followings [23–25]:
ACE: Forward 5´-ATTGCAGCCGGGCAACTTTT-3´; reverse 5´-CGCATTCTCCTCCGTGATGT-3´
ACE2: Forward 5´-GAGCCCATATGCCGACCAAA-3´; reverse 5´-TCTGCCTCCCCAAAAGGAAC-3´
AT1R: Forward 5´-GGCAAAGTCACCCGCATCAT-3´; reverse 5´-AATTTTTTCCCCAGAAAGCC-3´
AT2R: Forward 5´-GGAGCGAGCACAGAATTGAAAGC-3´; reverse 5´-TGCCCAGAGAGGAAGGATTGCC-3´
AT4R: Forward 5´-GATAAGATAGAAGTAGGGGAGA-3´; reverse 5´-CAATAGAGGTACAGTCACCA-3´
MasR: Forward 5´-CTCTCGGCTTTGTGGAGAAC-3´; reverse 5´-AGCGGAGTGAAGACCAAGAA-3´
Statistical analysis
All values are expressed as means±standard error of mean. Differences in hemodynamic variables over time between the control and the treatment groups were assessed using analysis of variance followed by Bonferroni multiple comparison test (Sigmaplot, Systat Software Inc). The significance level was set at P<0.05.
RESULTS
Changes in body weight and plasma renotropic hormones in experimental colitis
The mean body weight of SD rats receiving tap water increased from 209.4±2.0 g to 270.3±4.2 g in control group (n=10) and that of SD rats (n=10) receiving 5% DSS increased from 211.2±2.4 g to 252.1±4.4 g for 7 days (Fig. 1A). The gain of body weight in DDS-treated rats was less than that in control rats (40.9±2.4 g vs. 60.9±3.0 g, P<0.001). Hematocrit in experimental colitis group significantly was lower compared with control group (Fig. 1B). PRC was higher (57.1±6.7 ngAI/mL/hr vs. 31.0±3.5 ngAI/mL/hr, P<0.001) (Fig. 1C) and plasma ANP concentration was lower in experimental colitis (67.7±6.0 pg/mL vs. 108.5±11.3 pg/mL, P<0.001) than that in control group (Fig. 1D). There was an inverse correlation between PRC and ANP level (Fig. 1E). Changes in body weight has a positive correlation with plasma ANP level (Fig. 1F). and a negative correlation with PRC (y= –0.47x+ 51.7, r2=0.54; P<0.05). However, no significant correlation was observed between plasma ANP level and hematocrit (Fig. 1G).
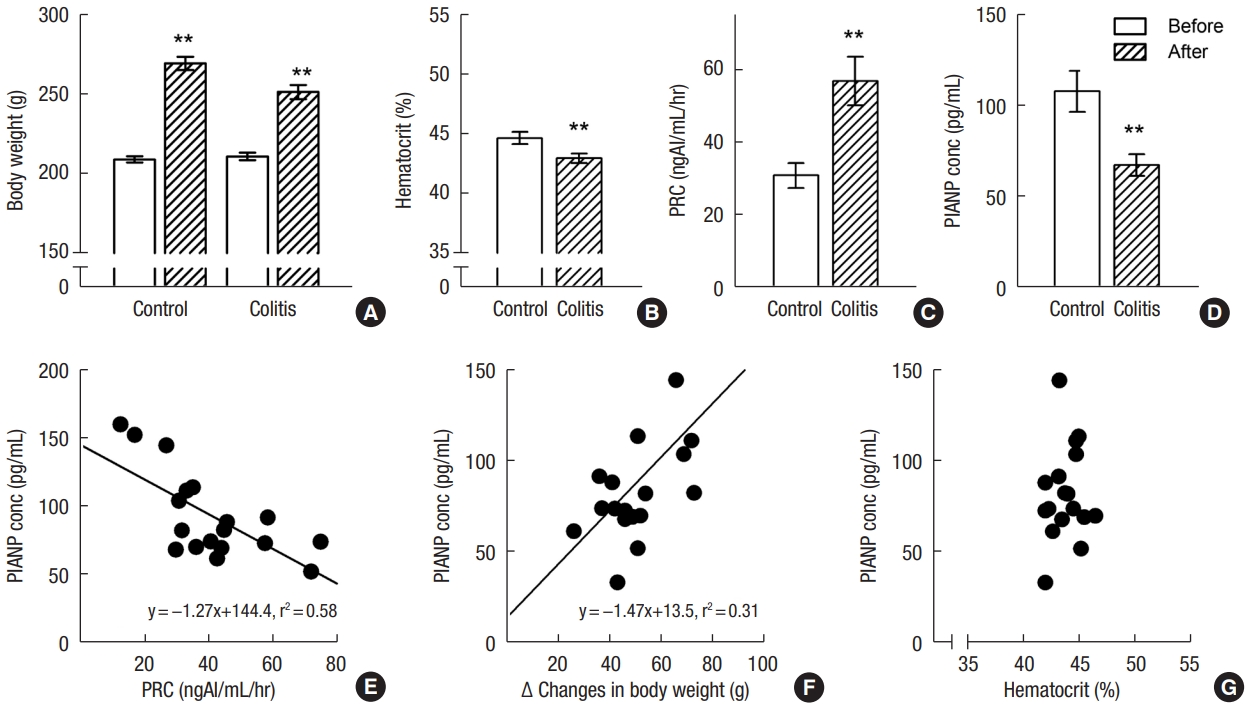
Comparison of body weight (A), hematocrit (B), and plasma concentration of renin (PRC; C), and atrial natriuretic peptide (ANP; D) in control and dextran sulfate sodium-treated rats. Correlation of plasma ANP (PlANP) concentration (conc) with PRC (E), body weight (F), and hematocrit (G). An inverse correlation between PRC and ANP level and a positive correlation between change in body weight and PlANP level were observed. Values are presented as mean±standard error of mean. **P<0.01 (vs. control rats).
Histopathological findings of colon in experimental colitis
DDS-treated rats showed marked macroscopic bleeding in the lumen of colon and bloody diarrhea. Fig. 2A–D shows representative histological images of H&E stained colon sections from both groups. In contrast to normal control rats (Fig. 2A, B), DSS-treated rats showed marked necrosis of the mucosa, inflammatory infiltration on the whole layers of intestinal wall, and marked edema on submucosa (Fig. 2C, D). Massive infiltration of inflammatory cells such as polymorphonuclear leukocytes, lymphocytes, and plasma cells was observed in the lamina propria and submucosa. Colonic weight and length were reduced by 17% and by 15%, respectively, as compared to the normal control group (Fig. 2E, F). The frequency and contractility of basal colonic motility in Taenia coli of DSS-treated rats appear to be low without significance as compared to control rats (Fig. 2G, H).
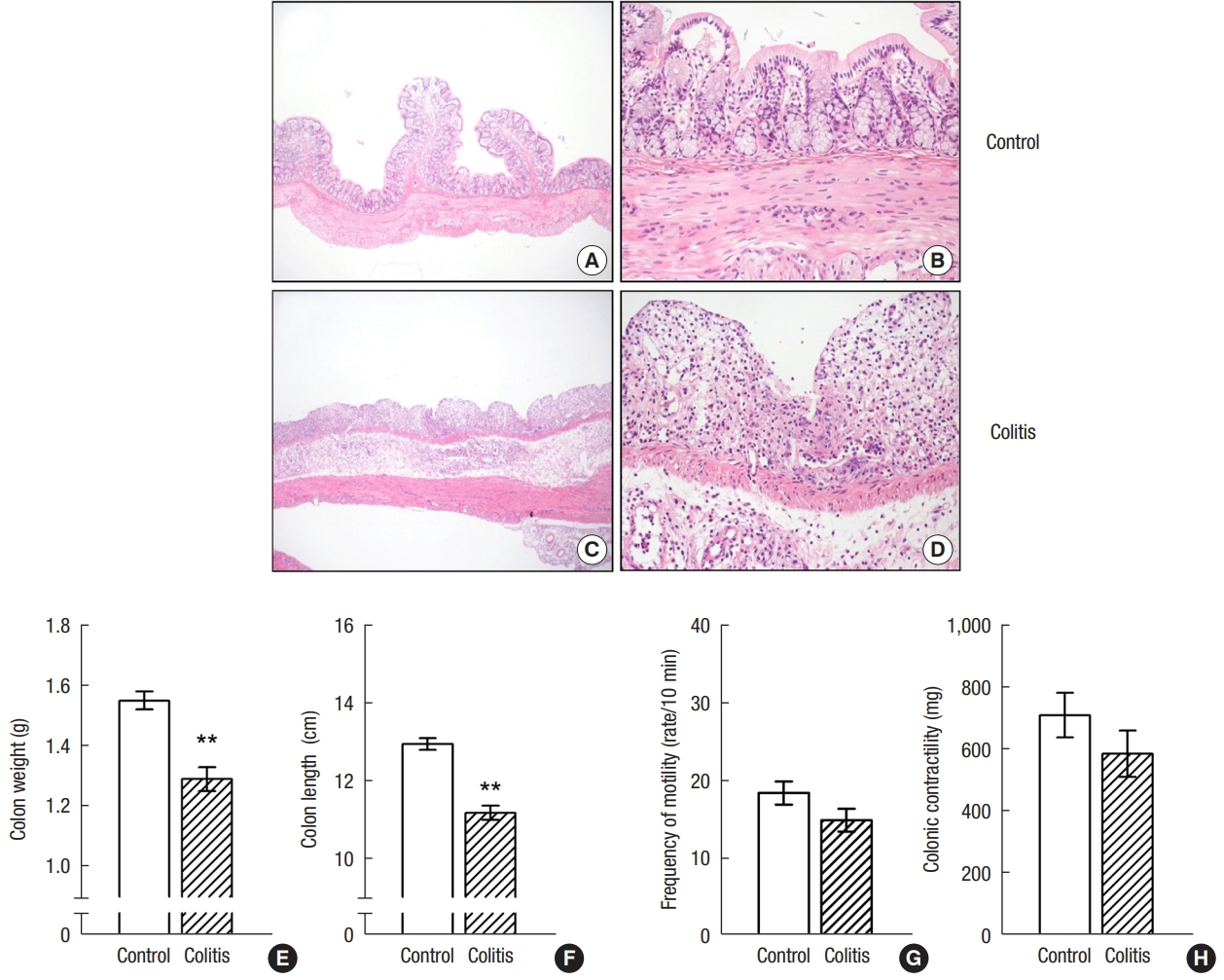
A representative histological images of hematoxylin-eosin (H&E)–stained colon sections in control (A, B) and dextran sulfate sodium (DSS)-treated rats (C, D). (A, C) H&E, ×2. (B, D) H&E, ×20. Rats with experimental colitis showed marked necrosis of the epithelium, intraluminal accumulation of mucous exudates, dilation of the crypts, and submucosal edema. Comparison of colon weight and length (E, F), and motility (G, H) in control and DSS-treated rats. Values are presented as mean±standard error of mean. **P<0.01 (vs. control rats).
Changes in messenger RNA expressions of colonic renin-angiotensin system in experimental colitis
In normal colon, mRNAs for ACE, ACE2, AT1R, AT2R, AT4R, and MasR were detected. In DSS-treated rat colon, the levels of ACE and ACE2 mRNA were not different from those in control rat colon (Fig. 3A, B). However, the expression of AT2R mRNA was significantly higher than that in control rat colon (Fig. 3C–F). The levels of AT1R, AT4R, and MasR mRNA were not different from those in control rat colon (Fig. 3C–F).
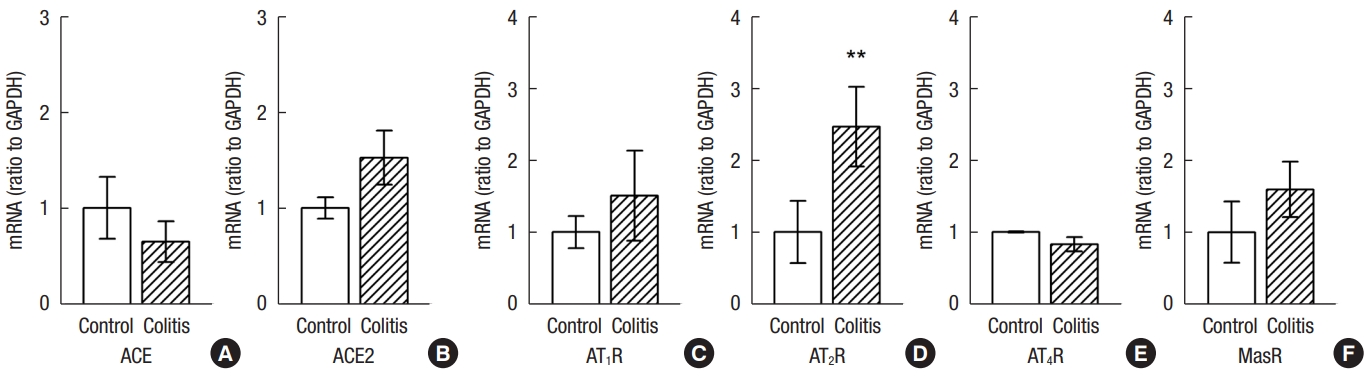
Comparison of expression of colonic angiotensin II converting enzyme (ACE) and ACE messenger RNAs (mRNAs) (A, B), and colonic angiotensin II receptor type 1 (AT1R), AT2R, AT4R, and MasR mRNAs (C–F) in normal and dextran sulfate sodium (DDS)-treated rats. The expression of AT2R was higher in DSS-treated rats than that in control rats. Values are presented as mean ± standard error of mean. **P<0.01 (vs. control rats).
Functional analysis of changes in colonic motility by angiotensins peptides
Fig. 4A shows a representative colonic motility by the addition of Ang II, Ang III, Ang IV, Ang-(1-9), and Ang-(1-7) in control rats. In control rats, a frequency of basal motility was abruptly increased followed by contraction and maintained for 5 minutes by 0.1-µM Ang II. Ang III, Ang IV, and Ang-(1-9) but not Ang-(1-7) (1 µM) increased frequency of basal motility. Fig. 4B and C shows the percent change in frequency and amplitude of basal motility as compared to control period. Percent increases in frequency by 1- and 3-µM Ang III were 19.8%±5.8% (n=5) and 43.5%±19.7% (n=5), respectively (Fig. 4B). Percent increases in frequency by 1- and 3-µM Ang IV were 42.3%±7.2% (n=7) and 64.3%±6.6% (n=5), respectively (Fig. 4B). Ang-(1-9) at a doses of 1 and 3 µM increased frequency of basal motility by 14.9%±3.7% (n=8) and 36.4%±0.7% (n=5), respectively. However, Ang-(1-7) at doses of 1, 3, and 10 µM (n=5) did not cause any significant changes in basal motility. Ang III and Ang IV increased amplitude of basal motility but Ang-(1-9) and Ang-(1-7) did not change it (Fig. 4C).

(A) A representative basal colonic motility by the addition of angiotensin (Ang) II, Ang III, Ang IV, Ang-(1-9), and Ang-(1-7) in control rats. Comparison of changes in frequency (B) and amplitude (C) of basal motility by Ang III, Ang IV, Ang-(1-9), and Ang-(1-7) in control rat colon. Values are presented as mean±standard error of mean of 5–8 rats. *P<0.05 (vs. low dose).
There was no significant difference in increase of colonic motility induced by Ang IV between DSS-treated rats and control rats (Fig. 5A). However, in DSS-treated rats, the responsiveness of increase in frequency of basal motility to Ang-(1-9) (n=8–10) was augmented (33.0%±3.9% vs. 14.9%±3.7% by 1 µM, P<0.01; 66.5%±6.1% vs. 36.4%±0.7% by 3 µM, P<0.01) (Fig. 5B).
DISCUSSION
In the present study, we found that mRNAs for ACE, ACE2, and Ang receptors exist in rat colon and colonic AT2R mRNA was upregulated in experimental colitis. Frequency of basal motility in Taenia coli was increased by Ang peptides except for Ang-(1-7) and the order of potency appears to be Ang II > > Ang IV> Ang III= Ang-(1-9). The response of increased basal motility to Ang-(1-9) was augmented in DSS-induced experimental colitis. These data suggest that Ang peptides are partly involved in the regulation of basal colonic motility.
Ang II is metabolized to the truncated forms such as Ang III, Ang IV, Ang-(4-8), Ang-(1-9), Ang-(1-7), and Ang-(1-5), which are found in the circulation with different concentrations. Several studies have been demonstrated that the truncated Angs peptides have different effects on blood pressure, cellular proliferation, and hormone secretion [26–30]. Especially, our lab also demonstrated direct effect of Angs peptides on ANP secretion with cardioprotection [6, 7, 24, 30–32]. The presence of all components of RAS in gastrointestinal tract [8–10] suggests to participate in the regulation of glucose, amino acid, fluid and electrolyte absorption and secretion, motility, inflammation, blood flow, and possibly IBD [10, 11]. Ang II in high concentrations has been reported to increase intestinal motility followed by contraction [33]. However, a few reports are found about the biological roles of Angs peptides such as Ang III, Ang IV, Ang-(1-9), and Ang-(1-7) in colon. In the present study, we found Ang III and Ang IV increased frequency and amplitude of colonic motility. Ang-(1-9) increased frequency but not amplitude of colonic motility. However, Ang-(1-7) did not cause any significant changes in frequency and amplitude. Ang II with low concentration (0.01–0.1 µM) increased colonic motility and then caused contraction. The order of potency of an increased frequency appears to be Ang II > > Ang IV> Ang III= Ang-(1-9). Even though Ang II is the major potent peptide of RAS, Ang III, and Ang IV have also some effects in increasing blood pressure and aldosterone secretion. In contrast, Ang-(1-7) as well as Ang-(1-9) appear to play an important role by counter-regulating Ang II actions [34, 35]. We also reported that Ang II suppressed ANP secretion [36] and Ang-(1-7) [7] and Ang-(1-9) [6] stimulated ANP secretion from isolated atria. Based on the above reports including our data, Angs’ effects and potency appears to be diverse depending on tissues and experimental conditions. Even though the potency of Angs peptides appears relatively weak as compared to Ang II, their direct effects on colonic motility suggest that Angs peptides may have a physiological significance in the regulation of colonic motility.
In this study, to understand the pathophysiological role of Angs peptides in IBD, DSS-induced colitis rat model was used. Clinical characteristics such as loss of body weight, bloody stools, and diarrhea shown in DSS-induced colitis were similar to other reports [19, 37, 38]. We also observed macroscopic bleeding in colon, necrosis and infiltration of lymphocytes in mucosa [37]. Weight gain and hematocrit were decreased but not prominent as compared to control rats. Change of body weight and hematocrit by DSS-induced colitis seems to be different in terms of experimental conditions such as body weight, feeding duration, and season. In the present study, the dose of DSS and feeding duration were similar to others [39] but we used rats weighing 200 g. According to Kamat et al. [39], colon length was decreased at day 5, and hematocrit was decreased at day 7 after DSS intake. Despite less prominent change in hematocrit in this study, plasma ANP concentration decreased markedly whereas PRC increased [19]. There were inverse correlations between ANP concentration, PRC, and change in body weight. Therefore, we suggest that changes in plasma renotropic hormones may be due to intestinal bleeding and diarrhea followed by a decreased body fluid volume.
It has been reported that the receptor for Ang III and Ang-(1-9) is AT2R, and the receptor for Ang IV and Ang-(1-7) is AT4R and MasR, respectively [4]. We have also reported direct effects of Ang II, Ang III, Ang IV, Ang-(1-9), and Ang-(1-7) on ANP secretion via their own receptors [6, 7, 24, 30–32]. We have measured mRNA levels for Ang-related receptor using RT-PCR in DSS-treated rat colon and control rat colon. Upregulation of AT2R mRNA in DSS-induced colitis was observed in this study. No significant differences in mRNA levels for ACE, ACE2, AT1R, AT4R, and MasR were found between both groups. Therefore, we tested whether the effects of Ang IV and Ang-(1-9) on intestinal motility may change in DSS-treated rats. The frequency and contractility of basal colonic motility in Taenia coli of DSS-treated rats appear to be low without significance as compared to control rats. An increased frequency of colonic motility by Ang-(1-9) augmented whereas that by Ang IV was similar to control rats. The reason for the augmentation of Ang-(1-9) effect may be due to an increased expression of AT2R mRNA. However, compared to an increased AT2R mRNA level, augmentation of Ang-(1-9) appears to be weak. Even though we do not know the reason at present, it is possible Ang-(1-9) may be involved in other roles such as regulation of fluid and electrolyte transport and blood flow in addition to the regulation of colonic motility.
In conclusion, these results showing some different effects of Angs peptides on colonic basal motility and the augmentation of basal motility to Ang-(1-9) in experimental colitis suggest that Angs peptides may be partly involved in the pathophysiological regulation of colonic motility.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
None.
Acknowledgements
The authors would like to express gratitude to Dr. Byung Mun Park and Dr. Weijian Li (Jeonbuk National University Medical School, Jeonju, Korea) and Professor Kuichang Yuan (Yanbian University, Yanji, China) for their help during the experiments.

