The albumin to globulin ratio is associated with clinical outcome in Japanese patients with ulcerative colitis
Article information
Abstract
Purpose
The albumin to globulin ratio (AGR) is a recognized chronic inflammation marker. No evidence regarding the relationship between AGR level and ulcerative colitis (UC) exists. The aim of this study was to evaluate the association between AGR and clinical outcomes among Japanese subjects with UC.
Methods
The study subjects consisted of 273 Japanese individuals with UC. AGR was divided into 4 categories (low, moderate, high, and very high). The definition of complete mucosal healing (MH) was based on the Mayo endoscopic subscore of 0. Clinical remission (CR) was defined as no rectal bleeding and no abnormally high stool frequency (<3 times per day).
Results
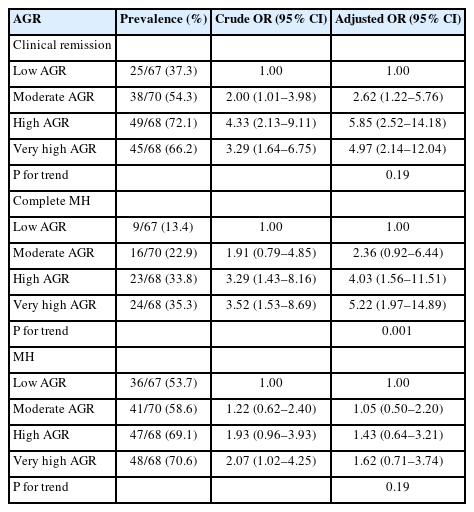
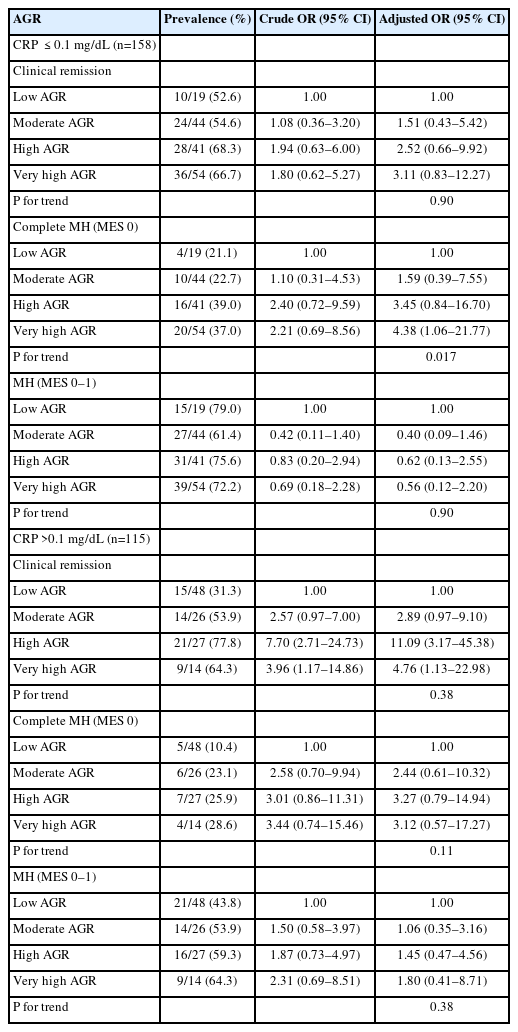
The percentage of MH was 26.4%. High AGR and very high AGR were significantly positively correlated with CR (adjusted odds ratio [OR], 5.85; 95% confidence interval [CI], 2.52–14.18 and adjusted OR, 4.97; 95% CI, 2.14–12.04) and complete MH (adjusted OR, 4.03; 95% CI, 1.56–11.51 and adjusted OR, 5.22; 95% CI, 1.97–14.89), respectively after adjustment for confounding factors (P for trend=0.001). Only in the low C-reactive protein (CRP) group (≤0.1 mg/dL), very high AGR was significantly positively correlated with complete MH but not CR (adjusted OR, 4.38; 95% CI, 1.06–21.77; P for trend=0.017). In the high CRP group, no correlation between AGR and complete MH was found.
Conclusion
Among Japanese patients with UC, AGR may be independently positively correlated with complete MH. In particular, among UC patients with low CRP, AGR might be a useful complementary marker for complete MH.
INTRODUCTION
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) characterized by a disease course involving relapses and remissions [1]. Mucosal healing (MH) is a short-term target in the clinical setting, as MH has been shown to be inversely correlated with relapse, hospitalization, colectomy, and colorectal cancer [2–7].
Strict control based on monitoring of inflammatory biomarkers is useful in managing MH [8]. Thus, the concept of the treat-to-target in UC has become widely accepted. Colonoscopy findings are the gold standard in assessing MH and fecal calprotectin is the most reliable marker for MH. However, repeated endoscopy and stool examination are extremely burdensome for patients.
Albumin and globulin are the 2 major components of serum proteins. The albumin to globulin ratio (AGR), calculated as serum albumin / (total protein – albumin), has been used as a marker for chronic inflammation. AGR has been reported to be a prognostic marker in patients with cancer, autoimmune diseases, heart failure, stroke, and sarcoidosis [9–18]. However, no evidence regarding the association between AGR and clinical outcomes among patients with UC exists.
C-reactive protein (CRP) is widely known as a serum inflammation marker and is associated with disease activity among patients with UC [19]. In a recent study, however, CRP failed to discriminate between endoscopic inflammation and MH [20]. The association between CRP and MH remains unclear [20–22]. Thus, an easily measurable serum biomarker for MH is still needed in the clinical setting.
Therefore, the primary purpose of this study was to analyze the association between AGR and clinical outcomes including MH in Japanese patients with UC, and the secondary purpose was to evaluate this issue after CRP stratification.
METHODS
Ethics statements
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) and was approved by the Institutional Review Board of the Ehime University Graduate School of Medicine (No. 1505011). Well-trained staff obtained written informed consent from all patients enrolled.
Study design
This study was a cross-sectional study using baseline data from a prospective cohort study. The study was registered at each hospital between 2015 and 2019.
Study population
The study subjects consisted of 387 Japanese patients with UC seen at the Department of Gastroenterology and Metabology of the Ehime University Graduate School of Medicine, and at several affiliated hospitals and clinics (Ehime Prefectural Central Hospital, Ehime Prefectural Niihama Hospital, Uwajima City Hospital, Saiseikai Matsuyama Hospital, Sumitomo Besshi Hospital, Saiseikai Imabari Hospital, and the OHASHI Clinic) in Ehime prefecture. All patients were diagnosed with UC according to endoscopic, radiological, histological, and clinical criteria. Consecutive outpatients and inpatients with UC who can understand our study were candidates. Patients with newly diagnosed UC and acute severe UC were also included in this cohort. However, even after being informed about this study and agreeing to participate, some subjects did not agree to undergo the colonoscopy and blood test; thus, some data are missing. After these 114 patients were excluded due to incomplete data, the final analysis sample in this study consisted of 273 patients.
Measurements
Information on endoscopic findings, age at onset of UC, medications, CRP, and AGR was collected using medical records. Blood samples were taken the morning after overnight fasting. Blood examination was performed when a colonoscopy was booked or when the colonoscopy examination was performed, and up to 2 months may have passed between the blood examination and the colonoscopy. Body mass index (BMI) was calculated as weight in kilograms divided by square of the height in meters.
Definition of clinical remission and mucosal healing
A certified endoscopist evaluated mucosal status by total colonoscopy. In patients with asymptomatic and/or mild UC, a colonoscopy was performed mainly once a year in spring or summer. In patients with moderate to severe UC, endoscopy was performed as required by the attending physician. In patients with newly diagnosed UC, colonoscopy was performed regardless of the severity of symptoms. Clinical remission (CR) was defined as no rectal bleeding and no abnormally high stool frequency (< 3 times per day). Complete MH and MH were defined as Mayo endoscopic score (MES) category 0 and MES 0–1, respectively [23]. One endoscopic specialist was responsible for evaluating MES, CR, complete MH, and MH, and was blinded to AGR.
Statistical analysis
AGR was divided into quartiles on the basis of the distribution of all study subjects. The 4 quartiles of AGR distribution in this study as follows: quartile 1 (n=67), < 1.281; quartile 2 (n=70), 1.281–1.483; quartile 3 (n=68), 1.483–1.643; quartile 4 (n=68), > 1.643. To investigate the exposure-response relationship between AGR and clinical outcomes, AGR level was classified into 4 categories: low AGR, < 1.281 (reference); moderate AGR, 1.281–1.483; high AGR, 1.483–1.643; and very high AGR, > 1.643. CRP was divided into 2 categories based on the median value of CRP: low CRP of ≤ 0.1 mg/dL and high CRP of > 0.1 mg/dL). Estimations of crude odds ratios (ORs) and their 95% confidence intervals (CIs) for CR, complete MH, and MH in relation to AGR were performed using logistic regression analysis. Multiple logistic regression analyses were used to adjust for potential confounding factors. Age, sex, steroid use, BMI, onset age, and anti-tumor necrosis factor α (TNF-α) preparation and disease extent (proctitis/non-proctitis) were selected as confounding factors. A receiver operating characteristic (ROC) curve was generated, and the area under the curve (AUC) was calculated to indicate the utility of the AGR for MH. The optimal Youden index-based cutoff point for MH was chosen to maximize the sum of sensitivity and specificity. Statistical analyses were mainly performed using the SAS software package ver. 9.4 (SAS Institute Inc). ROC curve, sensitivity, specificity, and cutoff value of AGR were analyzed using JMP 14.2 (SAS Institute Inc). All probability values for statistical tests were two-tailed, and a P-value of < 0.05 was considered statistically significant.
RESULTS
Table 1 shows the characteristics of the 273 study participants. The mean age and UC duration was 51.2 years and 9.0 years, respectively, and the percentage of male participants was 59.0%. Use of 5-aminosalicylates, prednisolone, thiopurines, and TNF-α monoclonal antibody preparations were 90.8%, 21.3%, 14.7%, and 5.9%, respectively. The percentage of MH was 26.4%, and the mean AGR was 1.461±0.291. In this cohort, median CRP was 0.099 mg/dL.
Table 2 shows crude and adjusted ORs and 95% CIs for CR in relation to AGR. Moderate, high, and very high AGR was positively correlated with CR (moderate AGR: crude OR, 2.00 [95% CI, 1.01–3.98]; high AGR: crude OR, 4.33 [95% CI, 2.13–9.11]; and very high AGR: crude OR, 3.29 [95% CI, 1.64–6.75]). After adjustment for confounding factors, the positive correlation between AGR and CR remained significant (moderate AGR: adjusted OR, 2.62 [95% CI, 1.22–5.76]; high AGR: adjusted OR, 5.85 [95% CI, 2.52–14.18]; and very high AGR: adjusted OR, 4.97 [95% CI, 2.14–12.04]). High AGR and very high AGR were positively correlated with complete MH (high AGR: crude OR, 3.29 [95% CI, 1.43–8.16] and very high AGR: crude OR, 3.52 [95% CI, 1.53–8.69]). After adjustment for confounding factors, high AGR and very high AGR were significantly positively correlated with complete MH (adjusted OR, 4.03 [95% CI, 1. 65–11.51] and adjusted OR, 5.22 [95% CI, 1.97–14.89]; P for trend=0.001). The positive correlation between AGR and MH disappeared after adjustment for these confounding factors.
The correlation between AGR and MH after CRP stratification is shown in Table 3. In the low CRP group (≤ 0.1 mg/dL), very high AGR was significantly positively correlated with MH but not MH and CR (adjusted OR, 4.38 [95% CI, 1.06–21.77]; P for trend=0.017). In the high CRP group (> 0.1 mg/dL), a positive correlation between high and very high AGR and CR was found (high: adjusted OR, 11.09 [95% CI, 3.17–45.38] and very high: adjusted OR, 4.76 [95% CI, 1.13–22.98]), while no correlation between AGR and MES found.
The AUC of AGR for identifying the MH was 0.613 (Fig. 1). When the cutoff value of AGR was 1.519, the sensitivity and specificity were 60.6% and 60.9%, respectively. The AUCs for identifying the MH in the low and high CRP groups were 0.657 and 0.529, respectively (Fig. 2). In the low CRP group, when the cutoff value of AGR was 1.538, the sensitivity and specificity were 60.0% and 87.1%, respectively.
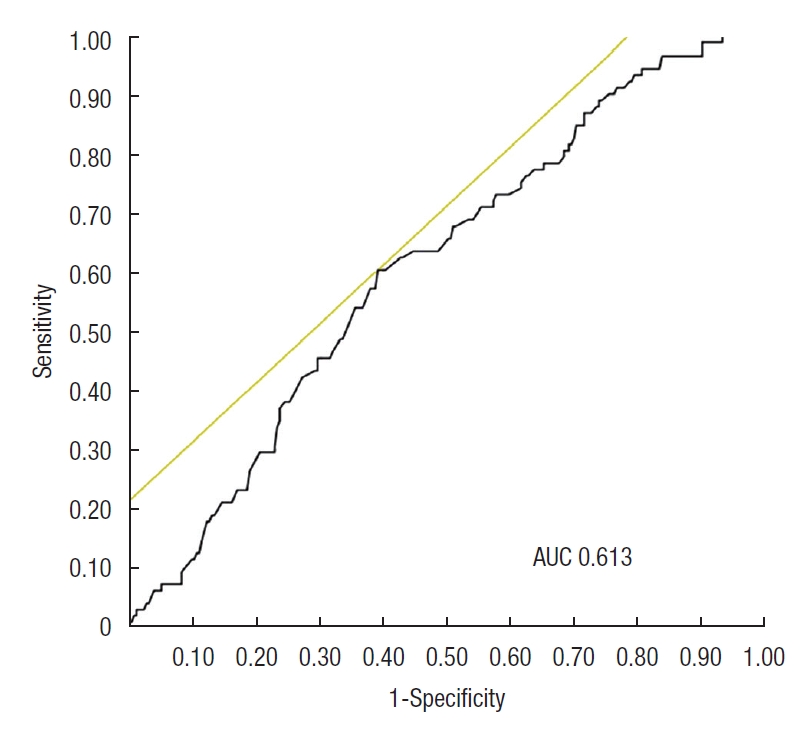
Receiver operating characteristic curve of albumin to globulin ratio (AGR) as a marker for mucosal healing (MH) defined as Mayo endoscopic score 0. The receiver operating characteristic curve of AGR as a marker for MH had an area under the curve (AUC) of 0.613. When the cutoff AGR level was set to 1.519, the sensitivity and specificity for MH were 60.6% and 60.9%, respectively.
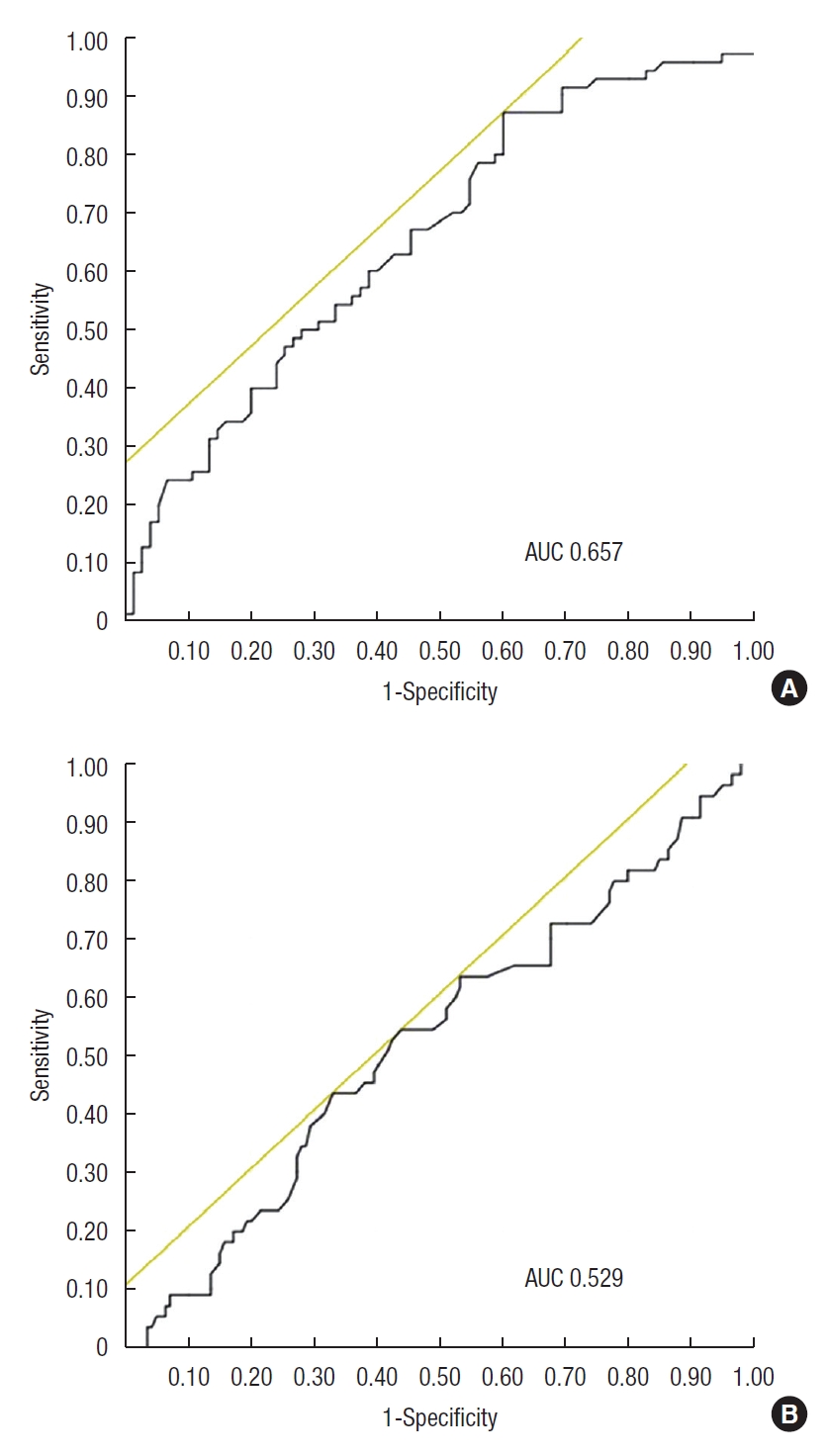
Receiver operating characteristic curve of albumin to globulin ratio (AGR) as a marker for mucosal healing (MH) after C-reactive protein (CRP) stratification. (A) In the low CRP group (≤ 0.1 mg/dL), the sensitivity and specificity for MH were 87.1% and 60.0%, respectively (cutoff value, 1.538) and the area under the curve (AUC) was 0.657. (B) In the high CRP group (> 0.1 mg/dL), the sensitivity and specificity were 43.9% and 54.6%, respectively (cutoff value, 1.517), and the AUC was 0.529.
In sensitivity analysis, the AGR value in patients with MH was significantly higher than in those without MH among patients receiving steroids and/or 5-aminosalicylates. The AGR value was not significant between MES 0 and 1 (1.538±0.237 and 1.465±0.297, respectively; P=0.077).
DISCUSSION
In the present study, AGR was independently positively correlated with complete MH among 273 Japanese patients with UC. AGR might be independently positively correlated with complete MH among UC patients with low CRP. This is the first study to show the association between AGR and MH in patients with UC.
Albumin and globulin are the 2 major components of serum proteins, and their levels are correlated with systemic inflammation [15–17, 24–28]. Low serum albumin could weaken the human immune system, thus increasing susceptibility to infection and resulting in cytokine-induced albumin suppression [24, 27]. AGR is less sensitive and less susceptible to measurement variability such as dehydration or fluid retention since it is a ratio rather than an absolute value [9].
AGR has been reported to be a prognostic marker in patients with cancer [13–15]. In a United States prospective cohort study of 534 patients with colorectal cancer, 4 years mortality in the lowest AGR was significantly higher compared to moderate and high AGR [13]. In a Turkish retrospective study of 240 lung adenocarcinoma patients, AGR value was positively correlated with mean survival time [14]. In a United States’ retrospective study of breast cancer, 5-year mortality in patients with high AGR was significantly lower than those with moderate and low AGR [15]. In a United States study of 999 patients with heart failure, 1-year all-cause mortality in low AGR was associated with high [16]. A similar association between AGR and cardiac and cerebrovascular events was found in a United States study and a Japanese study [17, 29]. In a United States study of 40 patients with sarcoidosis, AGR was associated with severity of sarcoidosis [18]. The findings of the present study support the notion regarding association between low AGR and worse prognosis.
CRP is associated with disease activity among patients with UC [19]. CRP was found to be a useful marker for MH in a United States study of 149 patients with UC [20]. However, CRP was not associated with MH in a German study of patients with UC [21]. In a Korean study, CRP was inversely correlated with MH, however, the sensitivity and specificity for MH were 50.5% to 50.3% and 85.1% to 87.2%, respectively [22]. The association between CRP and MH among patients with UC remains unclear. CRP is not useful marker for endoscopic activity in UC compared to Crohn disease [30]. The depth of inflammation in the gastrointestinal tract and the difference in interleukin-6 levels between UC and Crohn disease might complicate the use of serum markers for MH [31].
In patients with UC, the sensitivity and specificity of serum markers including AGR for clinical outcomes were not sufficiently high to justify using any of them alone. To assess MH using serum markers only, the use of combinations of serum markers should be investigated. Albumin and globulin can be measured inexpensively, quickly, and repeatedly in many medical institutions. AGR should be used as a complementary serum marker in UC patients with low CRP. AGR values may be useful to assess drug effects over a short period or in patients with asymptomatic UC.
Neutrophil-platelet ratio is associated with disease activity and endoscopic activity in patients with UC [32]. Additionally, neutrophil-platelet ratio is moderate accuracy for endoscopic activity. However, the association between neutrophil-platelet ratio and complete MH was still unclear. In our other analysis of this cohort, CRP to albumin ratio was independently positively associated with moderate to severe diseases activity (MES, 2–3) in patients with UC [33], while no association between CRP to albumin ratio and MH was found. Therefore, further study serum markers of UC activity or MH, as well as combinations of serum markers of disease activity are needed.
The underlying mechanism linking AGR and MH remains unclear, but there are several biologically plausible possibilities. Both serum albumin and globulin are recognized as chronic inflammation maker [15–17, 24–26]. In fact, in 43 patients with UC, CRP was found to be significantly inversely correlated with serum albumin level [30]. Because of intestinal tract inflammation, protein loss from the gut and malnutrition may influence serum albumin levels [34]. An inverse correlation between AGR and plasma TNF-α levels was found in community-dwelling Japanese women [35]. It was also found that α1 globulins levels are increased in the acute inflammation phase [36]. Chronic inflammation and protein loss in the intestinal tract in patients with UC might lower AGR via both TNF-α and globulin elevation and albumin decrease.
This study has several limitations. First, this was a cross-sectional analysis; therefore, we cannot conclude that there is a causal relationship between AGR and MH. Second, the exclusion rate for this cohort is high, resulting in a small sample size and the possibility of a type II error. Third, albumin, globulin, and CRP were measured with different kits in different patients, hospitals, and time periods. In Japan, however, the measurement methods for common laboratory data including albumin, globulin, and CRP are standardized, and all common laboratory data in this cohort were quality controlled using standard substances [37]. Fourth, the 2-month interval between endoscopy and blood tests might have caused some misclassification bias. However, non-differential misclassification might have led to underestimation of the association between AGR and complete MH. Fifth, one specialist was responsible for assigning the MES in this study. However, a central reading for endoscopic findings was not available. Finally, the subjects of the present study might be an unrepresentative sample of Japanese patients with UC. Nevertheless, the use of prednisolone and biologics were similar between the present study (20.8% and 6.1%, respectively) and a Japanese national study based on UC claims data in 2016 (15.5% and 9.0%, respectively) [38].
In conclusion, among Japanese patients with UC, AGR may be independently positively associated with MH. In particular, among UC patients with low CRP, AGR might be a useful complementary marker for MH.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
None.
AUTHOR CONTRIBUTIONS
Conceptualization: SY, SF, YY, ET, YI, Y Hiasa; Data curation: all authors; Formal analysis: SF; Investigation: SY, SF; Project administration: SF; Supervision: Y Hiasa; Writing–original draft: SY, SF; Writing–review & editing: all authors. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to thank Keitarou Kawasaki, Yuji Mizukami, Masayoshi Uraoka, Sanae Nakamura, Masamoto Torisu, Harumi Yano, Masato Murakami, Hino Harumi, Tomo Kogama and the ECNAD study group for their support.