- Search
| Ann Coloproctol > Volume 38(3); 2022 > Article |
|
Abstract
Purpose
We evaluated the oncological outcomes of bridge to surgery (BTS) using stent compared with surgery alone for obstructive colorectal cancer.
Methods
Consecutive patients who underwent curative resection for stages II to III obstructive colorectal cancer at our institution from January 2009 to March 2020, were registered retrospectively and divided into 43 patients in the BTS group and 65 patients in the surgery alone group. We compared the surgical and oncological outcomes between the 2 groups.
Results
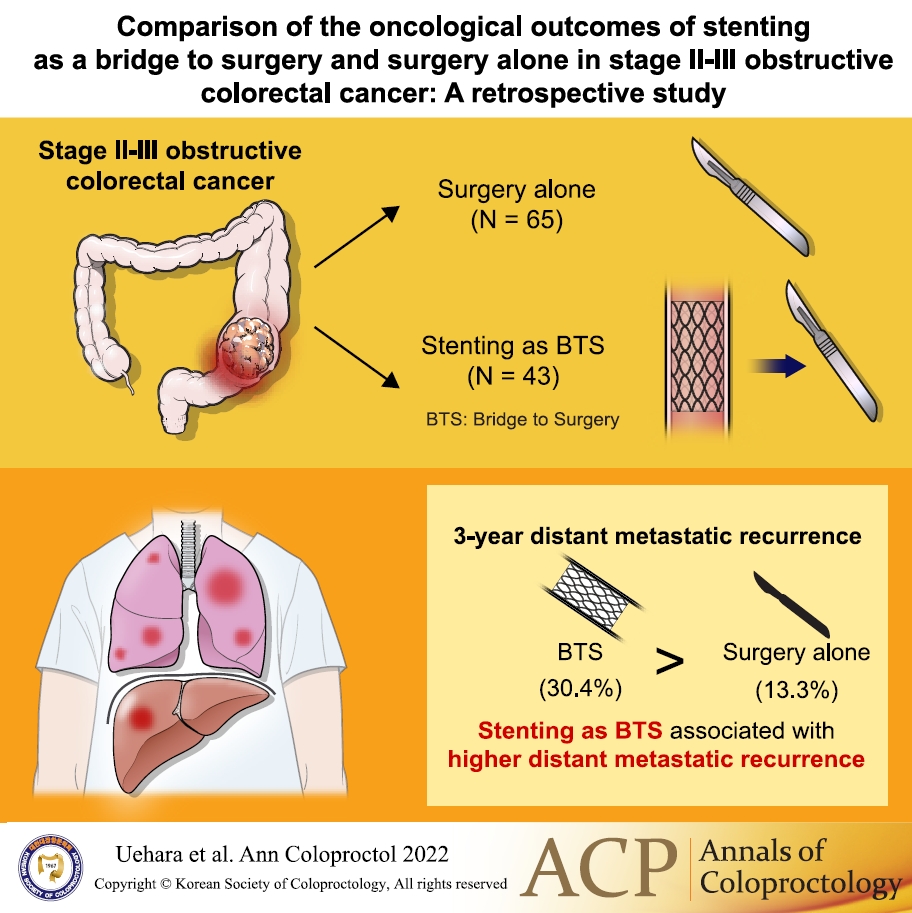
Stent-related perforation did not occur. One patient in whom the stent placement was unsuccessful underwent emergency surgery with poor decompression (clinical success rate, 97.7%). The pathological characteristics were not significantly different between the groups. The following surgical outcomes in the BTS group were superior to those in the surgery alone group; nonemergency surgery (P<0.001), surgical approach (P=0.006), and length of hospital stay (P=0.020). The median follow-up time was 44.9 months (range, 1.1–126.5 months). The 3-year relapse-free survival rates were 68.4% and 58.2% (P=0.411), and the overall survival rates were 78.3% and 88.2% (P=0.255) in the surgery alone and BTS groups, respectively. The 3-year locoregional recurrence rates were 10.2% and 8.0% (P=0.948), and distant metastatic recurrence rates were 13.3% and 30.4% (P=0.035) in the surgery alone and BTS groups, respectively.
Graphical Abstract
It has been reported that 8% to 16% of colorectal cancer patients develop bowel obstruction and require an emergency procedure to correct this issue [1-3]. Although patients with obstructive colorectal cancer generally undergo some type of emergency procedure, emergency surgery is associated with higher rates of mortality and morbidity compared with elective surgery [4]. In order to overcome this issue, a bridge to surgery (BTS) using stent for obstructive colorectal cancer was proposed. BTS using stent enables an elective resection in patients to potentially to avoid use of a stoma.
Initial systematic reviews have revealed a higher rate of laparoscopic surgery after stent placement, with lower morbidity rates, fewer temporary stoma creations, and higher primary anastomosis rates [5, 6]. Despite the short-term benefit of stent, stenting procedures might be associated with a higher rate of perineural invasion, increased potential for tumor cell dissemination, and increased risk of recurrence due to perforations associated with the stent or guidewire [7-10]. In a comparative study, Sabbagh et al. [11] used a propensity score analysis and found that BTS with stent was associated with worse overall survival compared with immediate surgery. In 2019, the Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines stated that obstruction relief by stent treatment as part of a BTS premised on curative surgical removal may also cause perforation and other adverse effects, which may worsen the long-term prognosis [12]. However, this has not been confirmed by recent meta-analyses [13]. In 2020, the European Society of Gastrointestinal Endoscopy guidelines recommended that stenting as a BTS be discussed as part of a shared decision-making process as a treatment option in patients with potentially curable left-sided obstructing colon cancer as an alternative to emergency resection [14]. We speculated that the locoregional recurrence rate would be worse in the BTS group compared with the surgery alone group.
To reveal the oncological outcome of stenting as a BTS for curable obstructive colorectal cancer, we hypothesed that the recurrence rate would be worse in the BTS group compared with the surgery alone group, and designed this retrospective cohort study at our institution.
We retrospectively evaluated and compared the surgical and oncological outcomes of BTS with stent for obstructive colorectal cancer and those of surgery alone. Consecutive patients who underwent curative resection for stages II to III obstructive colorectal cancer at Niigata City General Hospital were divided into the BTS group (treated from April 2012, when self-expandable metallic stent for malignant bowel obstruction was covered by insurance in Japan, to March 2020) and the surgery alone group (treated from January 2008 to March 2020). The surgery alone group included patients who did not undergo decompression and patients who underwent nonsurgical bowel decompression at the oral side of obstructive colorectal cancer and had a nasal ileus tube, nasogastric tube, or transanal decompression tube placed before curative resection. The patients who underwent preoperative bowel decompression procedures by stoma creation at the oral side of the cancer were excluded. The duration of decompression was defined as the time that elapsed between the nonsurgical bowel decompression procedures and curative resection. In this study, we defined emergency surgery as curative resection performed within 24 hours from the diagnosis of obstructive colon cancer.
In Japan, the indications for stent placement in cases of obstructive colorectal cancer are generally based on the ColoRectal Obstruction Scoring System (CROSS). The CROSS system was developed in 2012 by the Japan Colonic Stent Safe Procedure Research Group as a generally applicable technique for the assessment of colonic obstruction and stenosis; the CROSS system is published on the Research Group’s home page (Table 1 at http://colon-stent.com/). The colonic stents used in BTS for patients with CROSS scores between 0 and 1, and those used for palliative reasons in patients with scores between 0 and 3 are indicated appropriately [15]. At our institute, the indications for stent placement in each case of obstructive colorectal cancer were finally decided based on the CROSS scores, but the decisions were made not by surgeons but by gastroenterologists. Regarding stent placement, we treated malignant large bowel obstruction with either the WallFlex Colonic Stent (Boston Scientific Corp., Marlborough, MA, USA), HANAROSTENT Naturfit (Boston Scientific Corp.), or the Niti-S (Taewoong Medical, Gimpo, Korea). All procedures for stent placement were performed by a gastroenterologist.
Postoperative complications were defined according to the Clavien-Dindo classification system. Postoperative follow-up of colorectal cancer was performed according to the 2019 JSCCR guidelines for the treatment of colorectal cancer [12] and included outpatient visits at 3-month intervals during the first 3 years and visits at 6-month intervals thereafter until 5 years after curative resection; at a minimum, carcinoembryonic antigen levels were measured at each visit. Abdominal (liver) ultrasonography or computed tomography was recommended every 6 months for the first 3 years after resection and yearly thereafter. In addition, surveillance colonoscopy was performed at 1, 3, and 5 years after curative resection. In this study, locoregional recurrence was defined as recurrence in the anastomosis, regional lymph nodes, or peritoneum.
Continuous variables are expressed as medians (ranges). The Mann-Whitney U-test was used to compare continuous variables, and chi-square or Fisher exact tests were used to compare discrete variables. The Kaplan-Meier method and the log-rank test were used to compare the survival curves between groups. A comparison of recurrent probabilities was performed using a Cox proportional hazards model. All analyses were 2-sided, and P-values less than 0.05 were considered statistically significant. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria); more precisely, EZR is a modified version of R commander that is designed to add statistical functions that are frequently used in biostatistics.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or with comparable ethical standards. All participants have provided their written informed consent and the study was approved by Niigata City General Hospital Ethical Committee on human research (No. 20-008).
The patient characteristics are shown in Table 2. In all, 108 patients were retrospectively evaluated. The surgery alone group contained 65 patients, while the BTS group contained 43 patients. The median ages were 72 years (range, 31–92 years) in the surgery alone group and 69 years (range, 39–90 years) in the BTS group. Of the 65 patients in the surgery alone group, 58 (89.2%) had a CROSS score between 0 and 2, while of the 43 patients in the BTS group, 35 (81.4%) had a CROSS score between 0 and 2 (P=0.269). Twenty-three of 65 patients (35.4%) in the surgery alone group required preoperative nonsurgical bowel decompression while all 43 patients (100%) in the BTS group required bowel decompression, which was accomplished by the stent placement (P<0.001). The duration of the nonsurgical bowel decompression procedure before curative resection in the BTS group was significantly longer than that in the surgery alone group (9 days [range, 0–30 days] vs. 18 days [range, 10–67 days], P<0.001). In terms of tumor location, 25 of 65 patients (38.5%) had right-sided colorectal cancer in the surgery alone group, while 12 of 43 patients (27.9%) in the BTS group had right-sided cancer. However, this difference was not statistically significant (P=0.304). The pathological characteristics (e.g., tumor size, TNM category, histological type, venous invasion, and lymphatic invasion) were not significantly different between the 2 groups.
All stent placement procedures except one were clinically successful. The patient in whom the placement was unsuccessful underwent emergency surgery with poor decompression even after stent placement (clinical success rate, 97.7%). Stent-related perforations according to the pathological findings did not occur in any patients in the BTS group (stent-related perforation rate, 0%).
The surgical outcomes are shown in Table 3. One of the 43 patients (2.3%) in the BTS group required emergency surgery, which was significantly fewer than the 19 of 65 patients (29.2%) in the surgery alone group (P<0.001). In terms of the surgical approach used for curative resection, the number of patients in the BTS group who underwent laparoscopic surgery was significantly higher than that in the surgery alone group (P=0.006). Two of 43 patients (4.7%) in the BTS group and 11 of 65 patients (16.9%) in the surgery alone group required a stoma during curative surgery (P=0.072). Operative time in the surgery alone group was significantly shorter than that in the BTS group (187 minutes [range, 109−596 minutes) vs. 228 minutes [range, 138−343 minutes], P<0.001). No significant differences were observed in the number of postoperative days until flatus, stool discharge, or oral intake of food and water between the surgery alone group and the BTS group. The average length of postoperative hospital stay in the BTS group was 2 days shorter than that in the surgery alone group (11 days [range, 6−64 days) vs. 9 days [range, 6−30 days], P=0.020). Postoperative complications higher than grade 2 according to the Clavien−Dindo classification system occurred in 3 of 65 patients (4.6%) in the surgery alone group and in 4 of 43 patients (9.3%) in the BTS group, but this difference was not significant (P=0.375).
The characteristics of adjuvant chemotherapies used are shown in Table 4. The JSCCR guidelines recommended adjuvant chemotherapy in patients with high-risk stage II or stage III cancer [12]. For adjuvant chemotherapy, either oral 5-fluorouracil/leucovorin (FU/LV), oral 5-FU, capecitabine, mFOLFOX6 (5-FU/l-LV/oxaliplatin), CapeOX (capecitabine/oxaliplatin), or SOX (S-1/oxaliplatin) was selected in accordance with the patient’s condition. In all, 38 of 65 patients (58.5%) in the surgery alone group and 20 of 43 patients (46.5%) in the BTS group received any of the above regimens as adjuvant chemotherapy for 6 months.
The median follow-up time in the surgery alone group and the BTS group was 49.4 months (range, 1.1−126.5 months) and 37.8 months (range, 2.6−80.1 months), respectively. This difference was not statistically significant (P=0.079). The 3-year relapse-free survival rates were 68.4% and 58.2% (P=0.411), while the 3-year overall survival rates were 78.3% and 88.2% (P=0.255) for the surgery alone and BTS groups, respectively (Fig. 1). The 3-year locoregional recurrence rates were 10.2% and 8.0% in the surgery alone and BTS groups, respectively (P=0.948) (Fig. 2A), while the 3-year distant metastatic recurrence rates were 13.3% and 30.4% in the surgery alone and BTS groups, respectively (P=0.035) (Fig. 2B).
The Cox proportional hazards model analysis for recurrence in Table 5 showed no significant difference in any recurrences (hazard ratio [HR], 2.22; 95% confidence interval [CI], 0.95−5.19; P=0.066) and locoregional recurrences (HR, 1.05; 95% CI, 0.26−4.29; P=0.948), whereas a significant difference between treatment groups was found for distant metastatic recurrences (HR, 2.67; 95% CI, 1.03−6.91; P=0.043). Specifically, the number of patients in the BTS group who had liver recurrence was higher than that in the surgery alone group.
Currently, surgery is the standard of care for obstructive colorectal cancer. Several treatment procedures are feasible, ranging from diverting colostomy to colectomy with primary anastomosis and no stoma [16, 17]. The stent for obstructive colorectal cancer is an interesting alternative to emergency surgery because it combines the advantages of 1-stage surgery (1 operation and no stoma) and 2-stage surgery (treatment of the obstruction, correction of the patient’s comorbidities, and preparation for elective surgery) but avoids the drawbacks of surgical treatment (morbidity, mortality, length of hospital stays, and cost). Moreover, the feasibility of stent has been attested to by a meta-analysis, several reviews, and a randomized controlled trial [18-22]. Stent for obstructive colorectal cancer may be better than surgery in terms of the primary anastomosis rate, the stoma rate, morbidity, and cost [21, 23]. In this study, BTS using with stent in patients who presented with obstructive colorectal cancer was associated with better surgical outcomes. In a comparison of the surgery alone group and the BTS group, significant differences were found in the avoidance of emergency surgery, the presence of large wounds due to open surgery, and length of hospital stay. The median operative time was longer in the BTS group than in the surgery alone group (P<0.001), because the BTS group had a higher rate of laparoscopic surgery compared to the surgery alone group. Recently, a meta-analysis by Foo et al. [24] in 2019 reported that stents such as BTS had a lower risk of overall complications. A meta-analysis in 2018 and a propensity score-matched analysis in 2019 by Amelung et al. [25, 26] showed that permanent stomas rate in stent as BTS group were lower than in emergency surgery group. The better surgical outcomes in the BTS group might lead to an improvement in the postoperative quality of life in terms of good.
In the present study, BTS using stent for obstructive colorectal cancer with curative potential was associated with impaired mid-term oncological outcomes. When the 2 groups were compared, no significant differences were found in relapse-free survival, overall survival, and locoregional recurrence rates, but in contrast, a significant difference was found in distant recurrence. A meta-analysis by Ceresoli et al. [27] in 2017 reported that stents such as BTS had no adverse effects on the 3-year or 5-year mortality rates or on local recurrence. Recently, 2 meta-analyses revealed no significant difference between the stent group and the emergency surgery group in terms of overall survival, disease-free survival, and recurrence [13, 25]. In 2016, the multicenter, randomized controlled ESCO trial reported no difference in the 3-year overall survival and progression-free survival rates between the colonic stenting (BTS group) and the emergency surgery group [28].
When we designed this study, we speculated that the locoregional recurrence rate would be worse in the BTS group compared with the surgery alone group. However, in this study, the locoregional recurrence rate was not significantly different in both groups because no patient with a stent-related perforation was included in this study. If it is possible that stent-related perforations could occur in patients in the BTS group, the actual locoregional recurrence rate might be worse than that reported in this study. In 2013, the multicenter, randomized controlled Dutch Stent-In 2 trial reported that the locoregional or distant disease recurrence rate in the stent group was worse than that in the emergency surgery group and that the disease-free survival was worse in the subgroup with stent- or guidewire-related perforations [8]. The results of the present study suggested that stent placement for curable obstructive colorectal cancer might increase the rate of distant metastases even in cases without stent-related perforations. A meta-analysis by Foo et al. [24] in 2019 reported that a BTS stent was significantly associated with a greater chance of recurrence, especially systematic recurrence, although BTS was not associated with the 3-year disease-free survival or overall survival rate. In 2013, Sabbagh et al. [11] performed a retrospective comparative study with a propensity score analysis and found significantly poorer overall and 5-year survival rates than patients undergoing surgery only. These results suggested that stents may have an adverse effect on the long-term outcomes of colorectal cancer patients [11]. That study found a higher prevalence of synchronous distant metastasis in the stent placement group (37.5%) than in the surgery-only group (10.2%) and a lower success rate (81%) in the stent group. On the contrary, the present study limited patients to those without pathological stage IV and had a high clinical success rate (97.7%) and a low stent-related perforation rate (0%).
Takahashi et al. [29] reported that stent placement increased the plasma levels of cell-free DNA and circulating tumor DNA due to tumor manipulation. Maruthachalam et al. [7] reported that stent placement increased the cytokeratin 20 messenger RNA level in the peripheral blood and might be associated with increased recurrence of any type. In the present study, since a significant difference was observed in the distant recurrence rate, we could not deny the possibility that tumor compression by stent placement led to molecular biological effects that were associated with distant metastases. The Dutch Stent-In 2 trial reported that the liver recurrence rate was 12.5% in the stent group, which was worse than the 6.8% in the emergency surgery group [8].
These findings should be considered in several limitations. First, this study was limited by its small sample size and its retrospective, nonrandomized single-institution design. Thereby, the heterogeneity of the surgical strategy may have affected the prognostic factors. Although we limited patients to those with less than pathological stage IV cancer, the patient’s backgrounds were still heterogeneous. Second, the median follow-up time was relatively short, and a systematic difference was found in the observation period between the surgery alone group and the BTS group (median follow-up time was 49.4 and 37.8 months for the surgery alone group and BTS group, respectively). Patients in the BTS group were more recently treated than those in the surgery alone group. Given the advances in chemotherapy for recurrent colorectal cancer, there might have been a trend in the BTS group that the relapse-free survival rate was worse and the overall survival rate was better compared with the surgery alone group. Lastly, although endoscopic stent placement procedures were validated, stent devices used in this study had different lengths, types, and thickness and were obtained from different vendors.
In conclusion, BTS with stent seems to be an alternative procedure that can be used to avoid emergency surgery, large incisions, and a long hospital stay. Nevertheless, this study revealed that BTS with stent may be associated with a higher frequency of distant metastatic recurrence. Future research with larger sample size and a longer observation period is warranted to confirm the findings of the present study.
Fig. 1.
Kaplan-Meier survival curves of patients in the surgery alone group and the bridge to surgery (BTS) group. Numeric values show the 3-year relapse-free survival rates (A) and the 3-year overall survival rates (B) of the 2 groups.
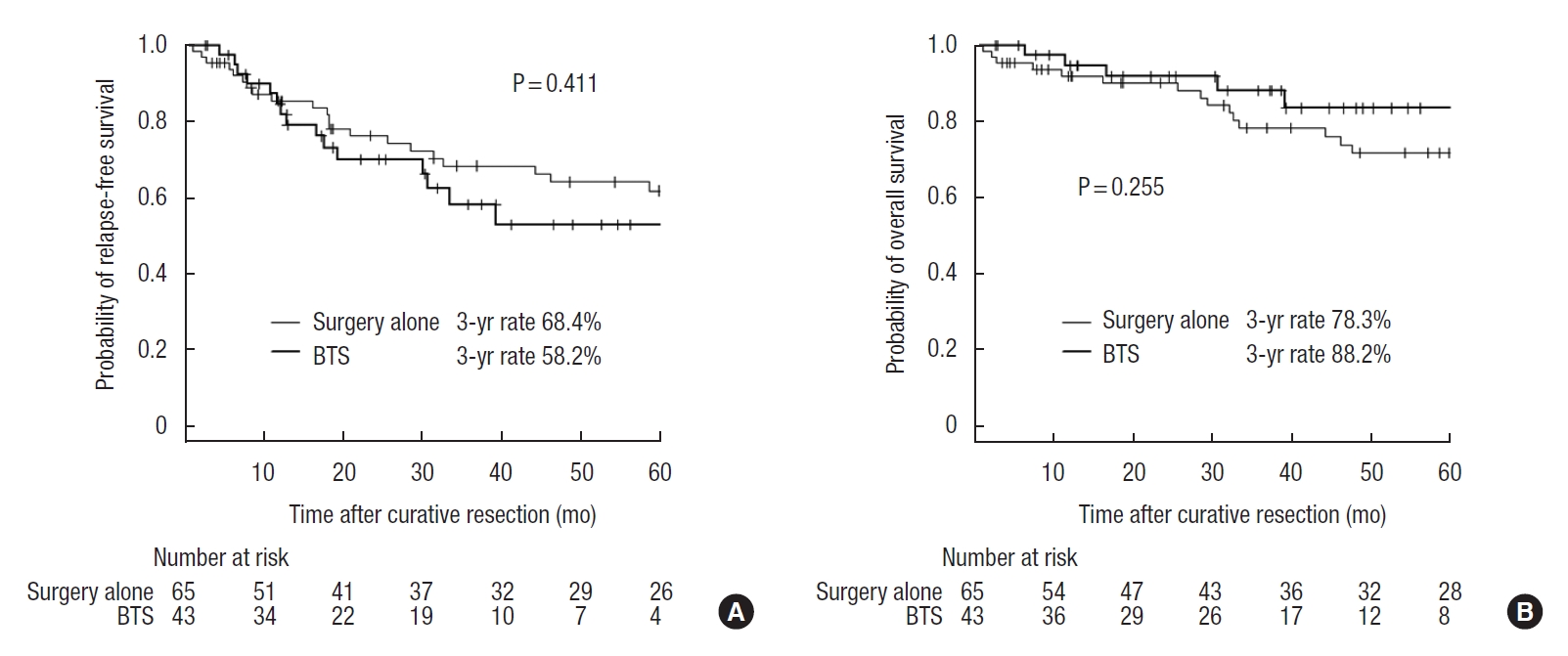
Fig. 2.
Kaplan-Meier curves of patients in the surgery alone group and bridge to surgery (BTS) group. Numeric values show the 3-year locoregional recurrence rates (A) and the 3-year distant metastasis rates (B) of the 2 groups.
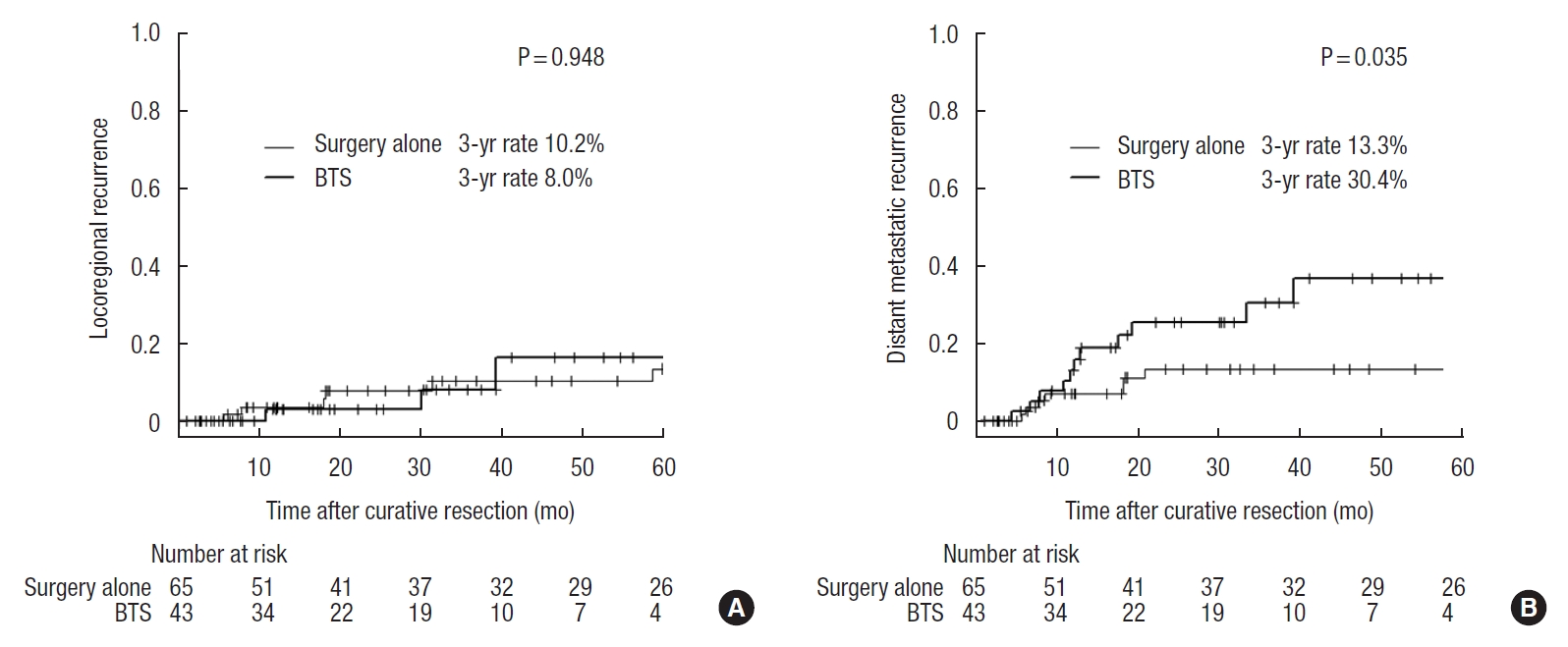
Table 1.
The ColoRectal Obstruction Scoring System (CROSS)
| Level of oral intake | Score |
|---|---|
| Requiring continuous decompressive procedure | 0 |
| No oral intake | 1 |
| Liquid or enteral nutrient | 2 |
| Soft solids, low-residue, and full diet with symptoms of stricture | 3 |
| Soft solids, low-residue, and full diet without symptoms of stricturea | 4 |
a Symptoms of stricture contain abdominal pain/cramps, abdominal distension, nausea, vomiting, constipation, and diarrhea which are related to gastrointestinal transit.
Adapted from Saida [15], according to the Creative Commons License.
Table 2.
Patients’ characteristics
| Characteristic | Surgery alone (n = 65) | Bridge to surgery (n = 43) | P-value |
|---|---|---|---|
| Sex | 0.422 | ||
| Male | 42 (64.6) | 24 (55.8) | |
| Female | 23 (35.4) | 19 (44.2) | |
| Age (yr) | 72 (31−92) | 69 (39−90) | 0.466 |
| Body mass index (kg/m2) | 21.5 (13.7−29.4) | 20.3 (14.6−28.4) | 0.496 |
| ASA PS classification | 0.674 | ||
| I | 21 (32.3) | 12 (27.9) | |
| II, III | 44 (67.7) | 31 (72.1) | |
| The score of CROSS | 0.269 | ||
| 0−2 | 58 (89.2) | 35 (81.4) | |
| 3, 4 | 7 (10.8) | 8 (18.6) | |
| Bowel decompressiona | 23 (35.4) | 43 (100) | < 0.001c |
| Duration of decompression (days)b | 9 (0−30) | 18 (10−67) | < 0.001c |
| Types of stent | - | ||
| WallFlex Colonic Stent | NA | 16 (37.2) | |
| HANAROSTENT Naturfit | NA | 7 (16.3) | |
| Niti-S | NA | 20 (46.5) | |
| Tumor location | 0.304 | ||
| Left-sided | 40 (61.5) | 31 (72.1) | |
| Right-sided | 25 (38.5) | 12 (27.9) | |
| Tumor size (cm) | 5.5 (2.5−13.5) | 5.6 (3.5−10.5) | 0.975 |
| pT category | 0.198 | ||
| pT2−3 | 49 (75.4) | 27 (62.8) | |
| pT4 | 16 (24.6) | 16 (37.2) | |
| pN category | 0.548 | ||
| pN0 | 38 (58.5) | 28 (65.1) | |
| pN1, 2 | 27 (41.5) | 15 (34.9) | |
| Histological type | |||
| Well or moderately differentiated tubular adenocarcinoma | 60 (92.3) | 43 (100) | 0.155 |
| Others | 5 (7.7) | 0 (0) | |
| Venous invasion | 0.542 | ||
| V0 | 26 (40.0) | 14 (32.6) | |
| V1, 2 | 39 (60.0) | 29 (67.4) | |
| Lymphatic invasion | 0.212 | ||
| Ly0 | 40 (61.5) | 32 (74.4) | |
| Ly1, 2 | 25 (38.5) | 11 (25.6) |
Values are presented as number (%) or median (range).
ASA, American Society of Anesthesiologists; PS, physical status; CROSS, The ColoRectal Obstruction Scoring System; NA, not applicable.
WallFlex Colonic Stent: Boston Scientific Corp., Marlborough, MA, USA; HANAROSTENT Naturfit: Boston Scientific Corp.; Niti-S: Taewoong Medical, Gimpo, Korea.
a Bowel decompression was defined as non-nonsurgical procedures by a nasal ileus tube, nasogastric tube or colonic stent.
Table 3.
Surgical outcomes
| Variable | Surgery alone (n = 65) | Bridge to surgery (n = 43) | P-value |
|---|---|---|---|
| Emergency surgery | 19 (29.2) | 1 (2.3) | < 0.001a |
| Surgical approach | 0.006a | ||
| Open | 41 (63.1) | 15 (34.9) | |
| Laparoscopic | 24 (36.9) | 28 (65.1) | |
| Stoma creation during curative resection | 11 (16.9) | 2 (4.7) | 0.072 |
| Number No. of dissection lymph nodes | 21 (3−113) | 25 (8−57) | 0.418 |
| Resection status of lymph node | 0.120 | ||
| D1 | 6 (9.2) | 0 (0) | |
| D2 | 15 (23.1) | 9 (20.9) | |
| D3 | 44 (67.7) | 34 (79.1) | |
| Operative time (min) | 187 (109−596) | 228 (138−343) | < 0.001a |
| Blood loss (g) | 80 (0−3,110) | 10 (5−1,430) | 0.362 |
| POD until gas or stool discharge | 2 (0−9) | 2 (0−18) | 0.736 |
| POD until oral intake starting | 4 (2−18) | 4 (3−20) | 0.918 |
| Postoperative hospital stay (days) | 11 (6−64) | 9 (6−30) | 0.020a |
| Postoperative complications | 18 (27.7) | 11 (25.6) | > 0.999 |
| CD grade I−II | 15 (23.1) | 7 (16.3) | 0.375 |
| CD grade III−V | 3 (4.6) | 4 (9.3) | |
| Ileus | 3 (4.6) | 4 (9.3) | |
| Intraperitoneal abscess | 4 (6.2) | 1 (2.3) | |
| Anastomotic leak | 2 (3.1) | 0 (0) | |
| Wound infection | 3 (4.6) | 3 (7.0) | |
| Cardiovascular event | 1 (1.5) | 0 (0) | |
| Cerebrovascular event | 1 (1.5) | 0 (0) | |
| Others | 4 (6.0) | 3 (7.0) |
Table 4.
Characteristics of adjuvant chemotherapy
Table 5.
Oncological outcomes for recurrence
| Variable | Surgery alonea (n = 65) | Bridge to surgery (n = 43) | HR (95% CI)b | P-value |
|---|---|---|---|---|
| Any recurrences | 10 (15.4) | 12 (27.9) | 2.22 (0.95−5.19) | 0.066 |
| Locoregional recurrences | 6 (9.2) | 3 (7.0) | 1.05 (0.26−4.29) | 0.948 |
| Anastomotic | 0 (0) | 1 (2.3) | ||
| Peritoneum | 5 (7.7) | 3 (7.0) | ||
| Pelvic lymph node | 1 (1.5) | 0 (0) | ||
| Distant metastatic recurrences | 7 (10.8) | 11 (25.6) | 2.67 (1.03−6.91) | 0.043c |
| Liver | 1 (1.5) | 6 (14.0) | ||
| Lung | 3 (4.6) | 3 (7.0) | ||
| Para-aortic lymph node | 2 (3.1) | 1 (2.3) | ||
| Ovary | 1 (1.5) | 1 (2.3) |
REFERENCES
1. Cheynel N, Cortet M, Lepage C, Benoit L, Faivre J, Bouvier AM. Trends in frequency and management of obstructing colorectal cancers in a well-defined population. Dis Colon Rectum 2007;50:1568–75.


2. Jullumstrø E, Wibe A, Lydersen S, Edna TH. Colon cancer incidence, presentation, treatment and outcomes over 25 years. Colorectal Dis 2011;13:512–8.


3. Winner M, Mooney SJ, Hershman DL, Feingold DL, Allendorf JD, Wright JD, et al. Incidence and predictors of bowel obstruction in elderly patients with stage IV colon cancer: a population-based cohort study. JAMA Surg 2013;148:715–22.



4. Tekkis PP, Kinsman R, Thompson MR, Stamatakis JD, Association of Coloproctology of Great Britain, Ireland. The Association of Coloproctology of Great Britain and Ireland study of large bowel obstruction caused by colorectal cancer. Ann Surg 2004;240:76–81.



5. Arezzo A, Passera R, Lo Secco G, Verra M, Bonino MA, Targarona E, et al. Stent as bridge to surgery for left-sided malignant colonic obstruction reduces adverse events and stoma rate compared with emergency surgery: results of a systematic review and meta-analysis of randomized controlled trials. Gastrointest Endosc 2017;86:416–26.


6. Atukorale YN, Church JL, Hoggan BL, Lambert RS, Gurgacz SL, Goodall S, et al. Self-expanding metallic stents for the management of emergency malignant large bowel obstruction: a systematic review. J Gastrointest Surg 2016;20:455–62.


7. Maruthachalam K, Lash GE, Shenton BK, Horgan AF. Tumour cell dissemination following endoscopic stent insertion. Br J Surg 2007;94:1151–4.


8. Sloothaak DA, van den Berg MW, Dijkgraaf MG, Fockens P, Tanis PJ, van Hooft JE, et al. Oncological outcome of malignant colonic obstruction in the Dutch Stent-In 2 trial. Br J Surg 2014;101:1751–7.

9. Yamashita S, Tanemura M, Sawada G, Moon J, Shimizu Y, Yamaguchi T, et al. Impact of endoscopic stent insertion on detection of viable circulating tumor cells from obstructive colorectal cancer. Oncol Lett 2018;15:400–6.



10. Kim HJ, Choi GS, Park JS, Park SY, Jun SH. Higher rate of perineural invasion in stent-laparoscopic approach in comparison to emergent open resection for obstructing left-sided colon cancer. Int J Colorectal Dis 2013;28:407–14.


11. Sabbagh C, Browet F, Diouf M, Cosse C, Brehant O, Bartoli E, et al. Is stenting as “a bridge to surgery” an oncologically safe strategy for the management of acute, left-sided, malignant, colonic obstruction? A comparative study with a propensity score analysis. Ann Surg 2013;258:107–15.


12. Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 2020;25:1–42.



13. Cao Y, Gu J, Deng S, Li J, Wu K, Cai K. Long-term tumour outcomes of self-expanding metal stents as ‘bridge to surgery’ for the treatment of colorectal cancer with malignant obstruction: a systematic review and meta-analysis. Int J Colorectal Dis 2019;34:1827–38.


14. van Hooft JE, Veld JV, Arnold D, Beets-Tan RG, Everett S, Götz M, et al. Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2020. Endoscopy 2020;52:389–407.


15. Saida Y. Current status of colonic stent for obstructive colorectal cancer in Japan; a review of the literature. J Anus Rectum Colon 2019;3:99–105.



16. Otchy D, Hyman NH, Simmang C, Anthony T, Buie WD, Cataldo P, et al. Practice parameters for colon cancer. Dis Colon Rectum 2004;4:1269–84.

17. Faivre J, Adenis A, Bretangne JF, Carpentier F, Durocher A, Debeco I, et al. Prévention, dépistage et prise en charge des cancers du côlon [Consensus conference. Prevention, diagnosis and treatment of colon cancer]. Gastroenterol Clin Biol 1998;22:205–26. French.

18. Dohmoto M. New method: endoscopic implantation of rectal stent in palliative treatment of malignant stenosis. Endosc Dig 1991;3:1507–12.
19. Sebastian S, Johnston S, Geoghegan T, Torreggiani W, Buckley M. Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol 2004;99:2051–7.


20. Khot UP, Lang AW, Murali K, Parker MC. Systematic review of the efficacy and safety of colorectal stents. Br J Surg 2002;89:1096–102.


21. Watt AM, Faragher IG, Griffin TT, Rieger NA, Maddern GJ. Self-expanding metallic stents for relieving malignant colorectal obstruction: a systematic review. Ann Surg 2007;246:24–30.



22. Zhang Y, Shi J, Shi B, Song CY, Xie WF, Chen YX. Self-expanding metallic stent as a bridge to surgery versus emergency surgery for obstructive colorectal cancer: a meta-analysis. Surg Endosc 2012;26:110–9.


23. Osman HS, Rashid HI, Sathananthan N, Parker MC. The cost effectiveness of self-expanding metal stents in the management of malignant left-sided large bowel obstruction. Colorectal Dis 2000;2:233–7.


24. Foo CC, Poon SH, Chiu RH, Lam WY, Cheung LC, Law WL. Is bridge to surgery stenting a safe alternative to emergency surgery in malignant colonic obstruction: a meta-analysis of randomized control trials. Surg Endosc 2019;33:293–302.


25. Amelung FJ, Burghgraef TA, Tanis PJ, van Hooft JE, Ter Borg F, Siersema PD, et al. Critical appraisal of oncological safety of stent as bridge to surgery in left-sided obstructing colon cancer; a systematic review and meta-analysis. Crit Rev Oncol Hematol 2018;131:66–75.


26. Amelung FJ, Borstlap WA, Consten EC, Veld JV, van Halsema EE, Bemelman WA, et al. Propensity score-matched analysis of oncological outcome between stent as bridge to surgery and emergency resection in patients with malignant left-sided colonic obstruction. Br J Surg 2019;106:1075–86.

27. Ceresoli M, Allievi N, Coccolini F, Montori G, Fugazzola P, Pisano M, et al. Long-term oncologic outcomes of stent as a bridge to surgery versus emergency surgery in malignant left side colonic obstructions: a meta-analysis. J Gastrointest Oncol 2017;8:867–76.