Analysis of the Incidence and Clinical Features of Colorectal Nonadenocarcinoma in Korea: A National Cancer Registry-Based Study
Article information
Abstract
Purpose
Although most colorectal malignancies are adenocarcinomas from mucosa, various types of malignant and benign tumors can develop. Due to extremely low incidence, little research has been conducted. The purpose was to assess incidence and compare it according to demographic factors.
Methods
Data from the Korea National Cancer Registry from 2007 to 2016 were used. The crude incidence, age-standard incidence rate (ASR) of colorectal nonadenocarcinomas were calculated.
Results
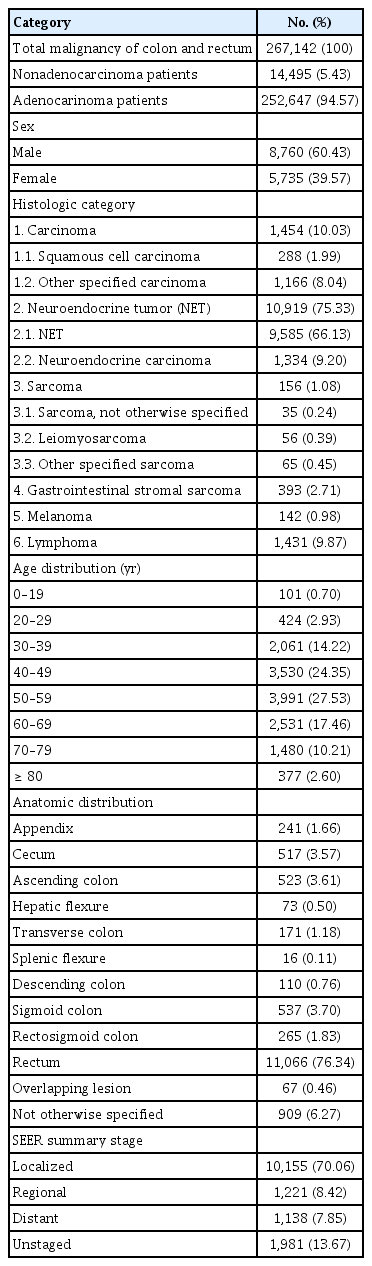
Over 11 years, there were 267,142 patients with colorectal malignancies. The patients of 14,495 (5.43%) were diagnosed with nonadenocarcinoma. The ASR was 2.52 per 100,000 in men and 1.56 in women. Lesions were classified according to histologic categories; neuroendocrine tumor (NET) was the most common malignancy (10,919 [75.33%]). Nonadenocarcinoma was the most common in 40s and 50s (40 to 49 years, 3,530 [24.35%]; 50 to 59 years, 3,991 [27.53%]). Lymphoma was high (54.46%) in patients in teenagers. Proportion of NET decreased with age and that of carcinoma increased with age. Carcinoma, sarcoma, and lymphoma were more common among men and melanoma was more common among women. The most common site was the rectum (11,066 [76.34%]). Lymphoma occurred more frequently in proximal colon. Melanoma, gastrointestinal stromal tumor, and NET occurred mostly in rectum. A total of 10,155 patients (70.06%) were classified as having localized disease.
Conclusion
This study is meaningful as it is the first study to examine incidence of colorectal nonadenocarcinoma. Differences in incidence of different lesions based on demographic factors were identified. This study will play a role in cancer prevention and diagnosis projects.
INTRODUCTION
Colorectal cancer has one of the highest incidences worldwide. It is the second most common malignancy in Europe and North America, following skin cancer [1, 2]. Additionally, the incidence of colorectal cancer is increasing in Asia, including Korea. It is the second most common cancer among men in Korea and the third most common among women [3]. Most colon cancers are adenocarcinomas originating from the colon mucosa. However, the colon is composed of various cell types other than mucosal cells. Therefore, various types of malignant and benign tumors, such as neuroendocrine tumors (NETs), gastrointestinal stromal tumors (GISTs), lymphomas, sarcomas, and lipomas may occur. However, since colorectal nonadenocarcinomas arise very infrequently, it is difficult to obtain a sufficiently large sample to perform comprehensive studies. Studies about nonadenocarcinoma have been rarely conducted for this reason, not only in Korea but also in other countries.
In the United States, the incidence of NET is 0.3 per 100,000 in the colon and 1.1 per 100,000 in the rectum [4], and a previous study found that neuroendocrine cancer in the colon accounts for 0.6% of total colon malignancies [5]. The incidence of squamous cell carcinoma of the colon is also very low, accounting for 0.01% to 0.85% of all colorectal cancers [6]; fewer than 100 cases of squamous cell carcinoma have been reported thus far [7]. Sarcoma that arises in the colon is reported to account for 0.1% of all colon malignancies [8]. Leiomyosarcoma accounts for 57.5% of colonic sarcomas and sarcoma accounts for 16.2% [9]. Colon melanoma is extremely rare; 13 cases have been reported, and there is no study about the incidence of colon melanoma [10]. Colonic lymphoma was reported to account for 0.1% to 0.6% of colorectal cancer [11]. There was no study about incidence of colorectal nonadenocarcinoma with nationwide data.
Some studies have examined colorectal nonadenocarcinoma, but studies about incidence are rare because of its low incidence. Therefore, the purpose of this study was to assess the incidence and identify the differences in the incidences of colorectal nonadenocarcinomas according to demographic factors using largescale data from the Korea National Cancer Registry.
METHODS
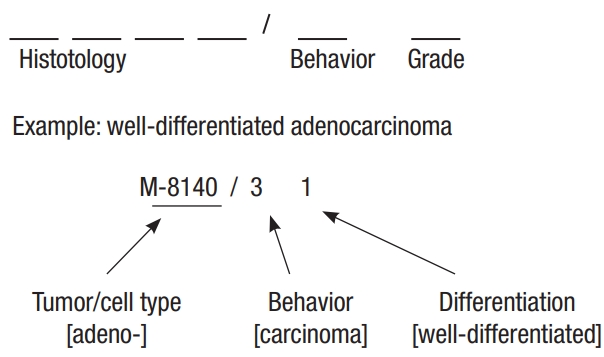
We used data from the National Cancer Registry, which is managed by the Korea National Cancer Center. We included the data of patients from 2007 to 2016 with “Korean Standard Classification of Diseases Diagnostic Code” of C18, C19, or C20, which indicate malignancy of the colon and rectum. Patients were classified pathologically using the Korean morphologic code (M code) [12]. Patients with an M code indicating adenocarcinoma were excluded. The M code is shown in Fig. 1. The M code consists of 5-unit numbers. The first 4 digits represent the histology of the neoplasm and the 5th digit represents its behavior. The M codes included in this study are listed in Table 1.
The purpose of this study was to investigate the incidence of colorectal nonadenocarcinoma. Therefore, most statistical analyses were performed to calculate the incidence and percentage. The variables used were sex, age, histological category, anatomical location, and SEER (Surveillance, Epidemiology and End Results) stage. To determine the incidence, the crude incidence and agestandard incidence were calculated. The population statistics in 2010 were used to calculate the age-standard incidence, and 95% confidence intervals were calculated. Cross-analysis was used to determine the characteristics of each histologic category according to age, sex, and anatomical location.
This study was approved by Institutional Review Board of National Health Insurance Service Ilsan Hospital (NHIMC 2018-07-007). The waiver of informed consent was granted for the collection of data from the existing medical records of patients.
RESULTS
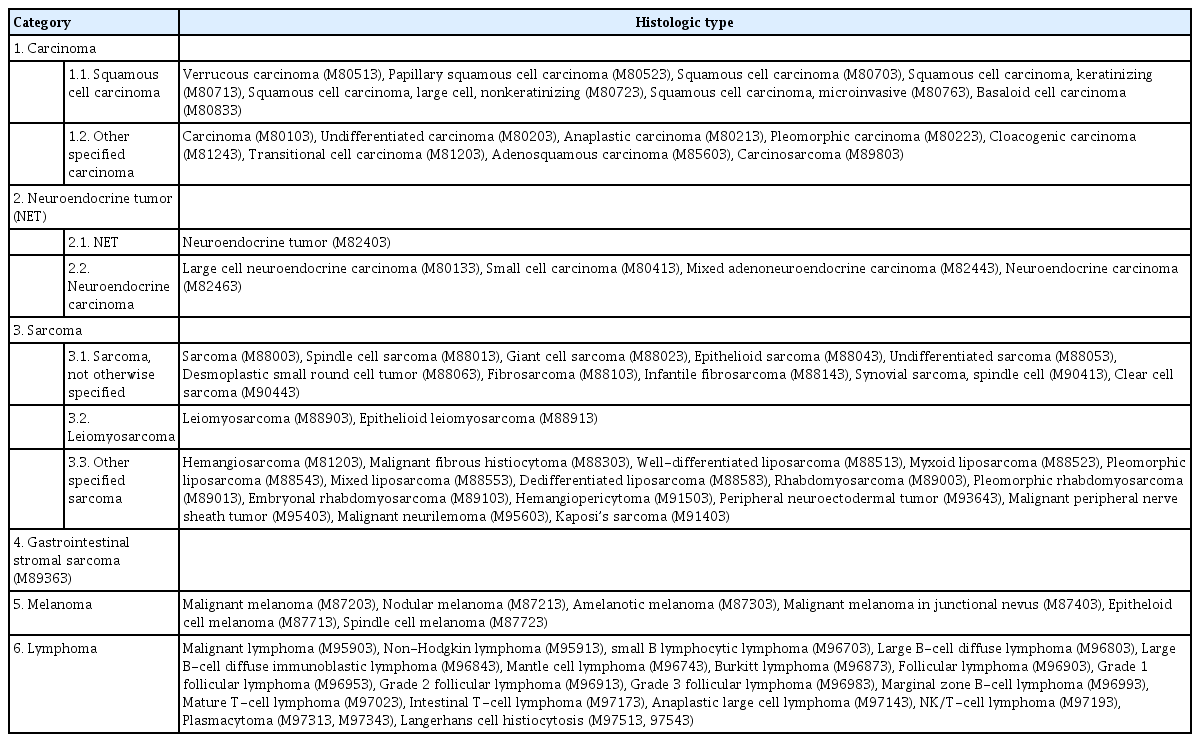
We first identified the total number of patients with nonadenocarcinoma of the colon and rectum from 2007 to 2016 in the Korea National Cancer Registry data. Over 11 years, 267,142 patients were diagnosed with colorectal malignancy. Among these, there were 14,495 (5.43%) nonadenocarcinoma patients. We divided the patients into 6 categories based on histologic diagnosis of nonadenocarcinoma. Each category and histologic diagnosis with M code are described in Table 1.
Demographic features of nonadenocarcinoma patients are described in Table 2. The majority of patients were men (8,760 [60.43%]). NET was the most common nonadenocarcinoma histology (10,919 [75.33%]), followed by carcinoma (1,454 [10.03%]), lymphoma (1,431 [9.87%]), GIST (393 [2.71%]), sarcoma (156 [1.08%]), and melanoma (142 [0.98%]). Patients in their 40s and 50s accounted for the highest percentage of patients (40 to 49 years, 3,530 [24.35%]; 50 to 59 years, 3,991 [27.53%]). Most nonadenocarcinomas occurred in the rectum (Table 2).
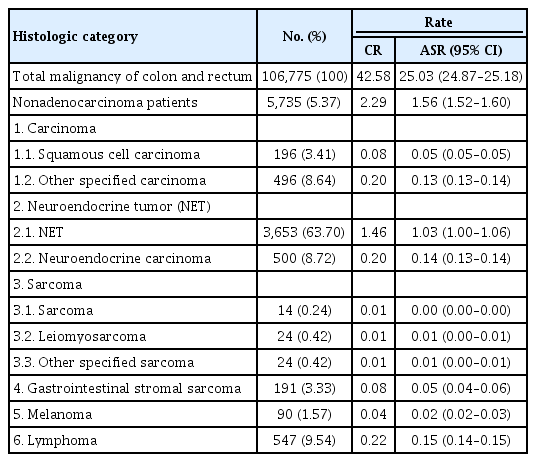
The number of patients and incidence of each nonadenocarcinoma were analyzed by sex (Tables 3, 4). The age-standardized incidence rates of nonadenocarcinoma were 2.52 per 100,000 men and 1.56 per 100,000 women. The proportion of nonadenocarcinoma among total colon malignancies was similar in both sexes but slightly higher in men (men vs. women, 5.46% vs. 5.37%). NET was the most common histology in both sexes. The proportions of the histologic categories in both sexes were similar, although most were slightly more common among men. However, melanoma tended to be more common among women (women, 90 [63.38%]; men, 52 [36.62%]).
We also analyzed incidence by age. The proportion of nonadenocarcinomas among total colorectal malignancies was higher in younger patients and decreased with increasing age (Table 5). NETs were the most common in most age groups. However, lymphoma was characteristically high in patients 10 to 19 years of age (54.46%); among patients aged 0 to 19 years old, lymphoma accounted for 54.46% of tumors. As patient age increased, the proportion of NET gradually decreased, and the proportion of carcinoma and melanoma increased. Carcinoma-except adenocarcinoma accounted for 46.68% of nonadenocarcinomas in patients 80 years and older.
We compared the incidence of nonadenocarcinomas according to the anatomical location of the colon (Fig. 2). Lymphoma was the most common lesion in the proximal colon, including the cecum and ascending colon (cecum, 420 [81.24%]; ascending colon, 250 [47.80%]). NET, which was the most frequent nonadenocarcinoma, was also the most frequent lesion in the appendix and rectum (appendix, 170 [70.54%]; rectum, 9,807 [88.62%]). Melanoma occurred in the rectum (136 [97.84%]), except for 3 cases each involving the sigmoid colon and rectosigmoid junction.
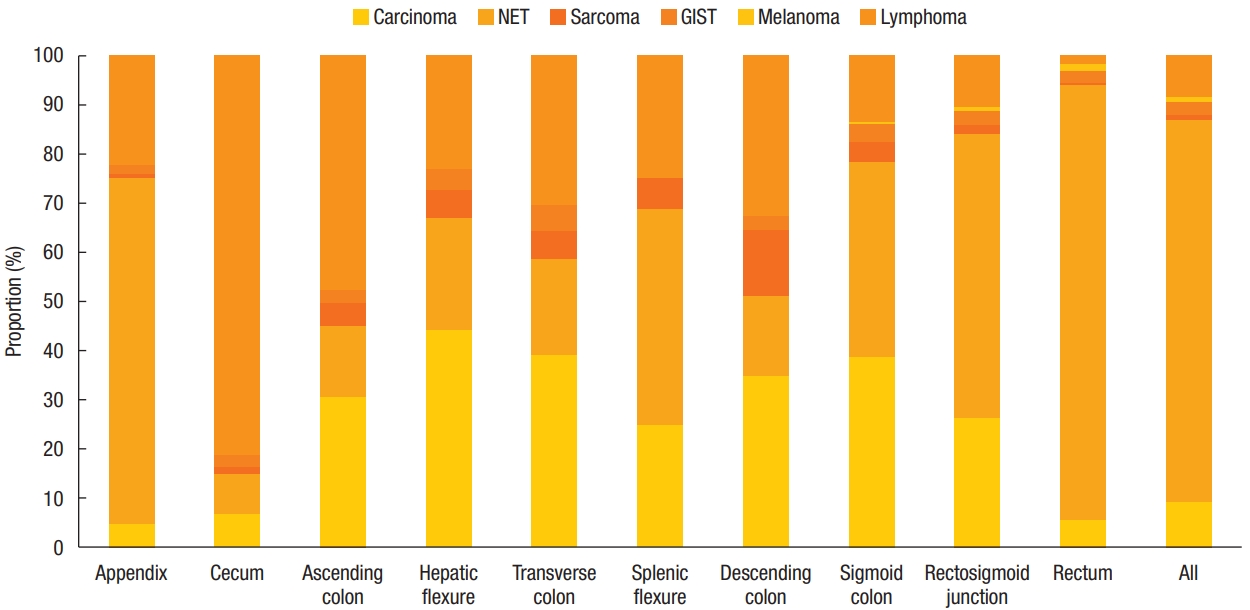
The proportion of nonadenocarcinoma by location. NET, neuroendocrine tumors; GIST, gastrointestinal stromal tumor.
The National Cancer Registry uses the SEER staging system. SEER stage is composed of localized disease, regional disease, distant disease, and unstaged disease. Among 14,495 patients, 10,155 patients (70.06%) were classified as having localized disease, 1,221 (8.42%) as having regional disease, and 1,138 (7.85%) as having distant disease. The SEER stage of each category of nonadenocarcinoma is shown in Fig. 3. NETs were mostly localized, but carcinomas, lymphomas, and melanomas were more commonly diagnosed as regional and distant disease. Due to the limitation of the National Cancer Registry, information about prognosis, including survival and recurrence, was not available. However, carcinomas, lymphomas, and melanomas were commonly diagnosed at advanced stages.
DISCUSSION
In this study, we analyzed the incidence of colorectal nonadenocarcinoma in Korea. Compared to adenocarcinoma, nonadenocarcinoma in Korea showed some characteristic features. Colorectal nonadenocarcinoma accounted for 5.43% of all colorectal malignancies. The incidence of nonadenocarcinoma in Korea was much lower than that of adenocarcinoma. The age-standardized incidence rates of colon adenocarcinoma in 2014 were 64.8 per 100,000 in men and 44.0 in women [13]. We also found that nonadenocarcinoma was relatively more frequent among younger patients than adenocarcinoma. The frequency of adenocarcinoma increases rapidly among patients in their 60s and is the highest among patients in their 60s, 70s, and 80s [13].
There has been little examination of the incidence and characteristics of colorectal nonadenocarcinoma. DiSario et al. [14] reported nonadenocarcinoma among 7,422 colon cancer patients in 1994; only 255 patients (3%) were diagnosed with nonadenocarcinoma. There were 75 patients (33%) diagnosed with squamous cell carcinoma, 74 patients (33%) with malignant NET, 37 patients (16%) with transitional cell-like tumor, 25 patients (11%) with lymphoma, 9 patients (4%) with sarcoma, and 2 patients (0.9%) with melanoma [14]. Because the most common colorectal malignancy is adenocarcinoma, most studies focus on adenocarcinoma. Due to the rarity of nonadenocarcinoma cases, they are mainly reported as case series. This study is meaningful in that it is almost the first study in Korea on the incidence and clinical characteristics of each colorectal nonadenocarcinoma.
Neuroendocrine carcinoma of the colorectum accounts for 0.6% of all colorectal malignancies [5] and occurs most frequently in patients from 60 to 89 years of age [8]. In this study, most NETs occurred in the rectum (93.07%). This is consistent with the fact that, in Asia, the rectum is a frequent location of NETs [15]. However, in western countries, NETs mostly occur in the proximal colon, including the cecum and ascending colon [16]. Neuroendocrine carcinoma has a male dominance and mean age at diagnosis around 50 years [16]. The incidence of NET in this study may be low compared to those found in other studies. The Korean Gastrointestinal Pathology Study Group suggested to diagnose colorectal grade 1 (G1) NETs with less than 1-cm size using D code, not C code [17]. Therefore, the diagnosis of malignant NET in Korea is conservative, and its incidence is less than that in other countries. In this study, we included G2 and G3 NETs, G1 NETs greater than 1 cm, and neuroendocrine carcinomas.
Squamous cell carcinoma accounted for 0.11% of the colorectal cancers in this study, which is similar to the results of a previous study that reported an incidence of 0.20% to 0.85% [7]. Squamous cell carcinomas are frequently located in the rectum because they can originate from the anal canal [18]. Adenosquamous carcinoma accounts for 0.03% to 0.18% of all colorectal malignancies [19], and cloacogenic carcinoma was reported in only 7 patients from 1977 to 2009 [20].
In previous studies, sarcoma accounted for 0.1% of all colorectal malignancies [8]. In this study, sarcoma accounted for 0.06% of all colorectal malignancies. Sarcoma is reported to be most frequently diagnosed in patients in their 60s and 70s [21]. Leiomyosarcoma in the colon is reported to account for 1% to 2% of all gastrointestinal malignancies [22]. A study using data from the American Cancer Registry showed that leiomyosarcoma was the most frequent sarcoma in the colon and rectum (57.5%), followed by sarcoma (16.2%) [23]. This study showed similar results.
Colorectal GIST accounts for 5% to 6% of all gastrointestinal tract GIST [24]. Colonic GIST shows male predominance, and the mean age at diagnosis is 67.5 years [25]. Due to the rarity of colorectal GIST, there are no reports about other clinical features of confined colorectal GIST.
In a previous study of 12 melanoma patients, there was a male predominance (1.4:1), and it was most common in the cecum and ascending colon [26]. However, in this study, there was a female predominance of melanoma and it was most common in the rectum. In this study, melanoma occurred only in the distal colon, including the sigmoid colon, rectosigmoid junction, and rectum.
Lymphoma occurred more frequently in the proximal colon, including the appendix, cecum, and ascending colon. Hangge et al. [27] reported that the most frequent location of lymphoma was the cecum, followed by the rectum and sigmoid colon. Lymphoma occurred more in men in this study. Chang [28] reported that primary colonic lymphoma showed male dominance. Primary colonic lymphoma accounts for 0.2% to 0.6% of all colorectal malignancies [29]. Most colonic lymphomas were reported to be B-cell lymphoma, but T-cell lymphoma has also been reported in Asia [30]. In this study, most lymphomas were B-cell lymphomas, although we also observed mature T-cell lymphoma and intestinal T-cell lymphoma.
This is the first nationwide analysis of colorectal nonadenocarcinoma in Korea. We identified differences in nonadenocarcinoma incidence based on demographic factors. There was a limitation in that the data only contained information on stage using SEER and not the TNM staging system; therefore, it was impossible to analyze detailed stage-specific features of each tumor. Further, as the National Cancer Registry showed only the incidence result, statistical analysis of each category was impossible. However, we were able to identify incidence based on different characteristics in nonadenocarcinoma as compared to adenocarcinoma; for example, nonadenocarcinoma was relatively more prevalent in younger patients than adenocarcinoma.
In conclusion, this study was the first to examine the incidence of colorectal nonadenocarcinoma in Korea. The characteristics of each nonadenocarcinoma, including carcinoma-except adenocarcinoma, NET, sarcoma, GIST, melanoma, and lymphoma, were identified. Cancer prevention and future management projects should be based on the basic characteristics of colorectal nonadenocarcinoma identified in this study.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.