Metronidazole in the Management of Post-Open Haemorrhoidectomy Pain: Systematic Review
Article information
Abstract
Purpose
Open haemorrhoidectomy is associated with significant postoperative pain. Metronidazole is commonly prescribed in the postoperative period as an adjunct to analgesia in pain management.
Methods
In our systematic review, studies were identified using PubMed/MEDLINE, Embase/Ovid and Cochrane Register of Controlled Trials databases. Studies were included if they were randomised controlled trials (RCTs) involving interventions with oral metronidazole at any dose over any time period. The primary outcome was pain score (visual analogue scale, VAS) after open haemorrhoidectomy. Secondary outcomes included time to return to normal daily activities, additional analgesia usage, and postoperative complications.
Results
Of 14 RCTs reviewed, 4 met inclusion criteria and were selected. The studies comprised 336 study subjects and 169 subjects were randomised to metronidazole while 167 were in the control group. There was a significant reduction in VAS across all time points, with maximal reduction seen on day 5 posthaemorrhoidectomy (mean difference, -2.28; 95% confidence interval, -2.49 to -2.08; P < 0.001). There was no difference in incidence of complications (P = 0.13). The Cochrane Risk of Bias Tool showed 3 of 4 of the studies had a risk of bias.
Conclusion
Metronidazole may be associated with decreased pain but there is insufficient evidence from RCTs to provide a strong grade of recommendation. Further RCTs are required.
INTRODUCTION
Open haemorrhoidectomy is considered the operation of choice for most third- and fourth-degree haemorrhoids [1]. However, it is associated with significant postoperative pain. Pudendal nerve blocks, opioid and nonopioid analgesics, creams, aperients, warm sitz baths, and metronidazole are used for pain management [2].
However, the mechanism by which metronidazole alleviates pain after haemorrhoidectomy is unclear. A popular theory is that metronidazole has a strong antibacterial effect on anaerobic gut organisms that may impair wound healing [3, 4]. De Paula et al. [5] reported that Escherichia coli is the most common organism found in haemorrhoidectomy wounds, with no obligate anaerobes identified. Conversely, another study found that transient bacteraemia is present in several patients posthaemorrhoidectomy and include obligate and facultative anaerobes [6]. Another suggested mechanism is that metronidazole has an antioxidant effect that promotes wound healing [3, 4].
Antibiotic bacterial resistance is increasing and the World Health Organisation considers it one of the greatest threats to global health [7]. Studies have shown that haemorrhoidectomy wounds heal without antibiotics [8]. There is ongoing debate about the utility of metronidazole after haemorrhoidectomies.
In this study, we evaluated the evidence in the literature regarding the role of metronidazole in reducing pain after open haemorrhoidectomy. We assessed time to resumption of work, complications, and analgesic requirements.
METHODS
This systematic review was registered with the PROSPERO (International Prospective Register of Systematic Reviews, registration number CRD42019126684). The Cochrane Handbook for Systematic Reviews of Interventions v5.1 [9] was consulted prior to commencing this review. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used to assist in writing this review [10].
The investigators designed and constructed the search strategy using relevant databases from commencement to 12 February 2019. Relevant studies were identified using a literature search of the following databases: PubMed/MEDLINE, Embase/Ovid and Cochrane Register of Controlled Trials. Clinical trial registries were also searched (www.clinicaltrials.gov) as well as other published systematic reviews and meta-analyses (including their reference lists) until 12 February 2019 to identify further studies. Two reviewers (AD and JT) individually assessed, included and excluded studies for this review.
Study selection
Studies were selected if they were randomised controlled trials (RCTs), with papers available in English, involving interventions with oral metronidazole at any dose over any time period. Patients who underwent open haemorrhoidectomy for internal haemorrhoids of all grades as well as external haemorrhoids were included. Trials could include additional interventions such as laxatives, topical anaesthetics, or glyceryl trinitrate, if an appropriate control group was included (i.e., both the intervention and control groups received them). Studies were excluded if they were observational, examined nonopen haemorrhoidectomy techniques, or if they examined topical metronidazole only.
Outcomes of interest
The primary outcome was postoperative pain on a visual analogue scale (VAS) at various time points. Secondary outcomes included time to return to work/normal daily activities, complications, and additional analgesic requirements.
Data extraction and quality assessment
Data were extracted independently by 1 reviewer (AD) and checked independently by the second reviewer (JT). Data was transcribed into a standardised form. Discrepancies were resolved by consensus between the 2 reviewers. If multiple publications of the same trial were identified, then the most recent publication of data was included. If study intervention subgroups included topical metronidazole, then these were also excluded from the analysis.
The risk of bias for each selected study was then analysed according to the revised Cochrane Risk of Bias Tool [11]. Two reviewers individually assessed allocation sequence, allocation concealment, blinding of participants and investigators, adherence to intervention regimen, use of appropriate analysis, and incompleteness of any data and reporting.
Data synthesis and statistical analysis
The summary statistics were derived from Review Manager ver. 5.3 (RevMan 5, Cochrane Collaboration, Oxford, UK) [12]. For continuous variables, meta-analysis was conducted using inverse variance either with a fixed-effects model (if I2 < 20), or a random-effects model (if I2 ≥ 20). Outcomes were recorded as mean differences (MD) with 95% confidence interval (CI). If continuous data were reported as a median and range, estimates of mean and standard deviation were calculated using a validated tool [13].
For dichotomous data, the Mantel-Haenszel method was used with a fixed effects model (if I2 < 20), or a random effects model (if I2 ≥ 20). Outcomes were recorded as odds ratios (ORs) with a 95% CI. P < 0.05 was considered statistically significant. The heterogeneity of studies was measured using I2.
RESULTS
A total of 144 studies were identified. After review of abstracts and full texts, 14 RCTs were identified. Four trials [3, 14-16] met inclusion criteria and were included in the meta-analysis. The majority of RCTs were not included as they either examined nonopen haemorrhoidectomy techniques and/or nonoral forms of metronidazole. The search and selection process for the RCTs is depicted in a PRISMA flow diagram (Fig. 1).
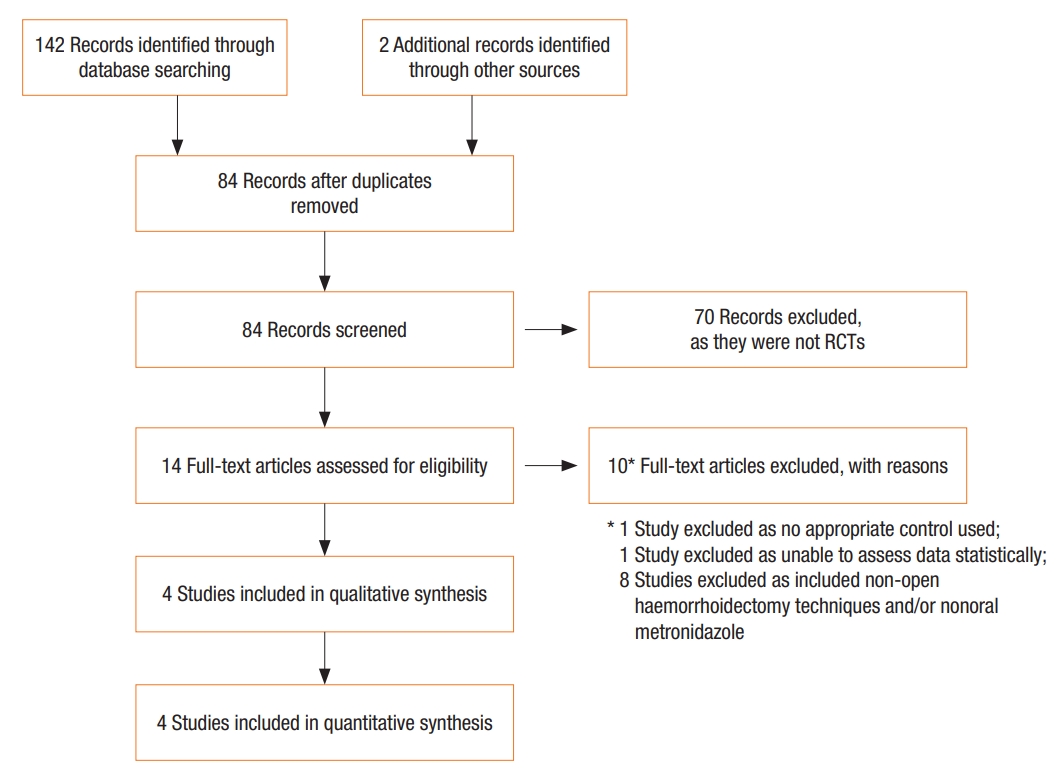
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram. RCT, randomized controlled trial.
Study characteristics
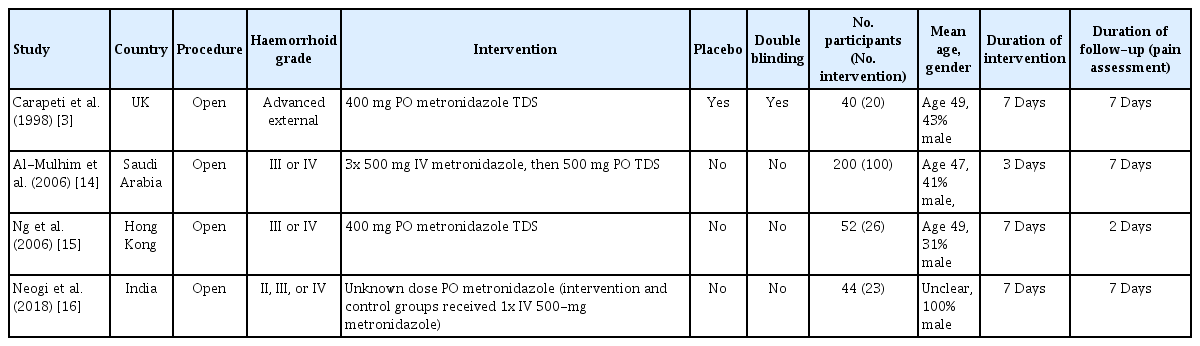
The study characteristics are summarised in Table 1. A total of 336 participants from the 4 RCTs were included in our analysis; 169 subjects were randomized to metronidazole and 167 were in the control group.
The trial of Carapeti et al. [3] involved patients with “advanced” external haemorrhoids only. Duration of treatment (oral metronidazole versus placebo tablets) and follow-up was 7 days.
The study of Al-Mulhim et al. [14] included patients with grades III and IV internal haemorrhoids. Patients in the intervention group received 3 doses of intravenous metronidazole before being prescribed oral metronidazole (versus the control group which received no antibiotics). Duration of intervention was 3 days, with a follow-up period of 7 days.
The trial of Ng et al. [15] involved patients with grades III and IV internal haemorrhoids only. Duration of intervention (oral metronidazole) was 7 days. However, pain assessment was only performed on the first 2 postoperative days and included assessment of pain at time of first bowel motion.
The trial of Neogi et al. [16] included patients with grades II, III and IV internal haemorrhoids. They did not report the oral metronidazole dose (duration was 7 days). There was a third trial arm that included topical metronidazole and was therefore excluded from our analysis. VAS was assessed on days 0, 1, 3, and 7. This study did not document a standard post-operative regimen (including analgesia) for the intervention and control groups. Both the intervention and control groups in this study received one dose of intravenous metronidazole 500 mg at the time of surgery. Duration of follow-up was 7 days.
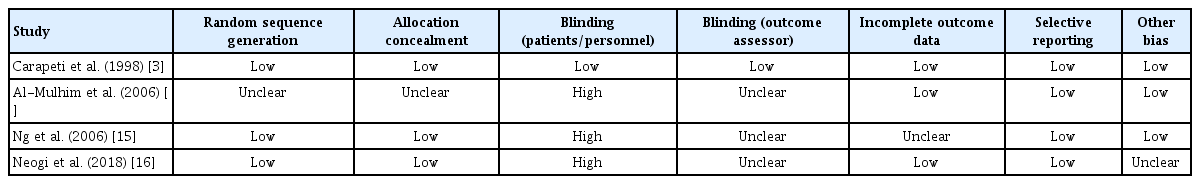
Risk of bias
The Cochrane Risk of Bias Tool [11] was used to assess each of the studies and is summarised in Table 2. Overall, 3 out of 4 studies [14-16] had high risk for bias. Only the study of Carapeti et al. [3] study was double-blinded, with the rest having no blinding (Table 2).
Study results
Postoperative pain
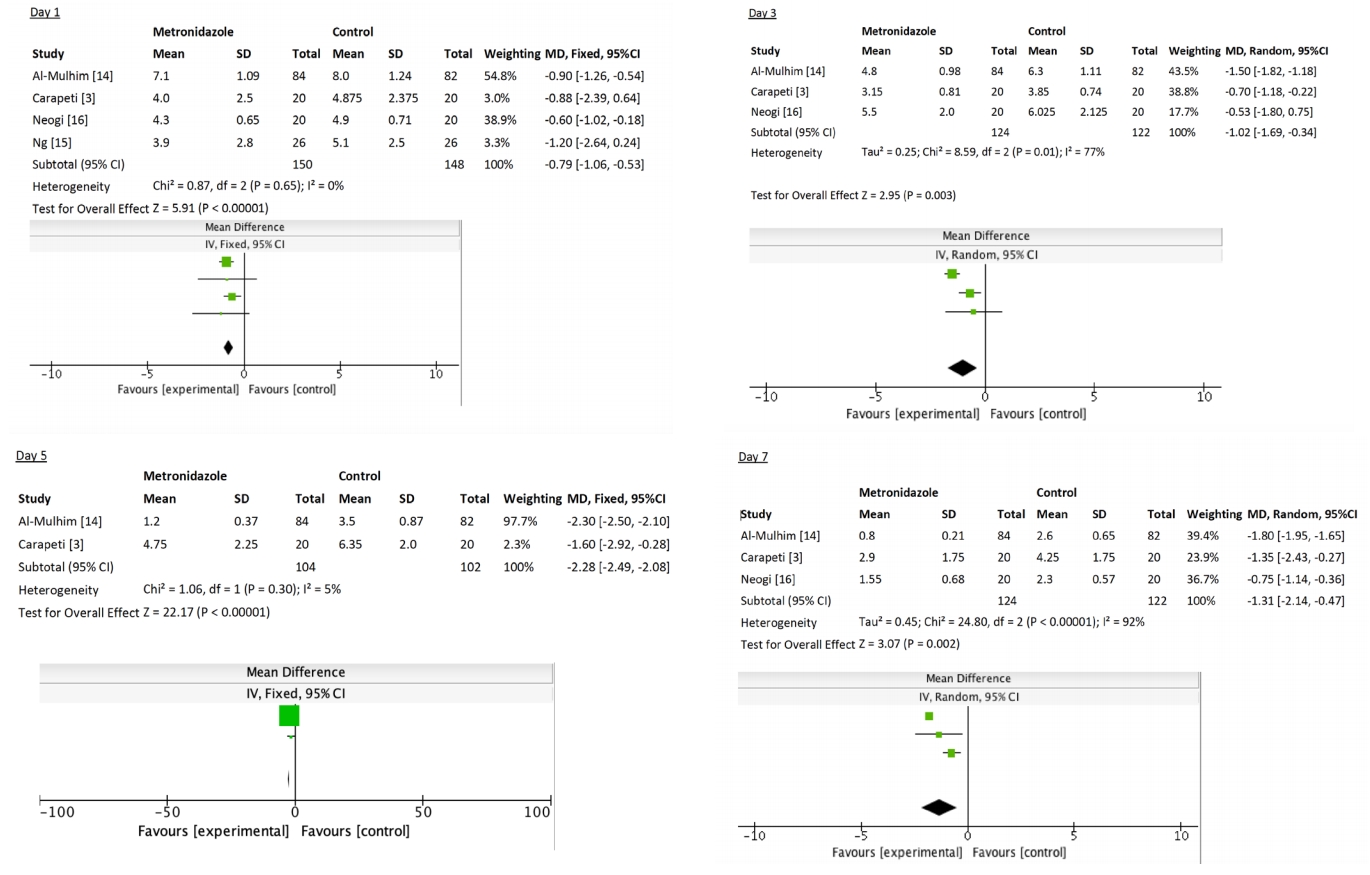
There was variation in the time points when the VAS was recorded for patients across all trials. Overall, there was a significant reduction in VAS across all time points (MD, -1.12; 95% CI, -1.4 to -0.83; P < 0.001) but with considerable heterogeneity (I2 = 90%, P < 0.001).
We also performed further subanalysis of VAS each day postoperatively. There was a consistent reduction in pain at each time point for patients who received metronidazole, with the greatest reduction in pain seen on day 5 (MD, -2.28), as listed: day 0 (MD, -0.39; 95% CI, -0.68 to -0.1; P = 0.009), day 1 (MD, -0.79; 95% CI, -1.06 to -0.53; P < 0.001), day 2 (MD, -0.98; 95% CI, -1.28 to -0.67; P < 0.001), day 3 (MD, -1.02; 95% CI, -1.69 to -0.34; P = 0.003), day 4 (MD, -1.43; 95% CI, -2.10 to -0.76; P < 0.001), day 5 (MD, -2.28; 95% CI, -2.49 to -2.08; P < 0.001), day 6 (MD, -2.00; 95% CI, -2.17 to -1.82; P < 0.001), and day 7 (MD, -1.31; 95% CI, -2.14 to -0.47; P = 0.002) (Fig. 2).
Ng et al. [15] also reported on pain associated with first bowel motion posthaemorrhoidectomy and found decreased pain in those who received metronidazole (P = 0.005).
Return to normal daily activities
Three of the studies [3, 14, 15] assessed time to return to work/normal daily activities. There was an overall trend toward a shorter time to return to work favouring metronidazole, however, this did not reach statistical significance (MD, -2.16; 95% CI, -5.07 to 0.75; P = 0.14).
Effect on analgesia consumption
All 4 trials reported on the effect of metronidazole versus placebo on analgesia requirements. The methods of reporting this, and analgesia requirements, differed between trials. Both Carapeti et al. [3] and Al-Mulhim et al. [14] commented on the number of patients requiring additional analgesia – overall a reduction in the number of patients requiring additional analgesia was found favouring metronidazole (OR, 0.45; 95% CI, 0.24–0.83, P = 0.01).
Ng et al. [15] and Neogi et al. [16] examined the actual number of additional analgesic tablets consumed. There was a trend toward fewer tablets consumed favouring metronidazole, but this did not reach statistical significance (MD, -3.04; 95% CI, -6.75 to 0.67; P = 0.11).
Adverse events
Two of the trials [14, 15] reported adverse events that occurred between metronidazole and control groups. There were no differences found in adverse events reported by the metronidazole and control groups (OR, 0.26; 95% CI, 0.04–1.51; P = 0.13). Commonly reported adverse events included urinary retention and constipation. The study of Al-Mulhim et al. [14] study reported on postoperative wound complications, with 2 patients (2%) in the metronidazole group and 16 patients (16%) in the control group having an unhealed wound at 6 weeks. All wounds had completely healed by 3 months.
DISCUSSION
This systematic review evaluates the role of oral metronidazole in decreasing postoperative pain following open haemorrhoidectomy. There appears to be a reduction in pain associated with metronidazole use. However, this finding is significantly limited by a lack of quality trials published. Carapeti et al. [3] was the only trial that was double-blinded; the rest were not blinded and were associated with a risk of bias. However, the study of Carapeti et al. [3] consisted of only 20 subjects randomized to each arm (oral metronidazole versus control). This study demonstrated a significant reduction in posthaemorrhoidectomy pain on day 5 (MD, -1.6; P = 0.004), day 6 (MD, -1.75; P = 0.02), and day 7 (MD, -1.35; P = 0.006). There were no significant differences in pain on days 1 through 4.
On pooled analysis, this review demonstrated a significant reduction in VAS at each time point. Yet there was no significant difference in return to work/normal daily activities between the metronidazole and control groups (P = 0.08). Therefore, whilst there may have been decreased pain, this did not necessarily translate into an improvement in overall outcome.
The trials included in this review had limited duration and follow-up. Posthaemorrhoidectomy patients can describe significant pain several weeks postoperatively [17], yet none of the trials examined pain more than 7 days postprocedure. The study of Ng et al. [15] only examined patients for the first 2 days postoperatively. While the differences in pain between the metronidazole and control groups were statistically significant across all time points, this difference was less than 1.0 on the VAS on 3 of the days (days 0, 1, and 2).
Two of the trials [14, 16] experienced some participant drop-out, although the rates were acceptable. In the trial of Al-Mulhim et al. [14], 84% of metronidazole participants and 82% of control participants completed the study. In the study of Neogi et al. [16] 87% of metronidazole participants and 95.2% of control participants completed the study. Neither of these trials analysed their data on an intention to treat basis. No studies assessed degree of compliance with trial medication.
It should be noted that there were initially 14 RCTs that we identified that could have been included in our meta-analysis. Eight RCTs were excluded as they either examined nonopen haemorrhoidectomy techniques and/or nonoral forms of metronidazole. We also excluded the trial of Di Vita et al. [18] trial as there was no appropriate control group (they included topical glyceryl trinitrate and metronidazole in the same treatment group without an appropriate control). Given that topical glyceryl trinitrate may also have an analgesic effect, we excluded this study in order to avoid confounding our results. We also had to exclude the trial of Ebied et al. [19], as their VAS data was unfortunately presented in a format that prevented us from analysing and pooling the data. The remaining 4 trials that we were able to include in our analysis had considerable heterogeneity (I2 = 90%, P < 0.001), and a limitation of our study was the small number of study subjects involved (336 total participants). However, we believe limiting our review to open haemorrhoidectomy only strengthened our analysis by reducing any potential confounding factors.
There have been 3 systematic reviews [20-22] published in recent years assessing the analgesic effects of metronidazole in posthaemorrhoidectomy patients. While these reviews have pooled more patients, all 3 reviews included both open and closed haemorrhoidectomies, which is not generalizable to open haemorrhoidectomy alone. The study of Wanis et al. [20], which reviewed oral metronidazole across 5 studies examining open and closed haemorrhoidectomy techniques, concluded that metronidazole was not associated with a significant reduction in pain on pooled analyses. However, on subanalysis they did find lower reported pain scores on day 1 (standardised mean difference [SMD], -0.87 ± 0.44; 95% CI, -1.73 to -0.015; P = 0.046) and day 4 (SMD, -1.43 ± 0.71; 95% CI, -2.83 to -0.037; P = 0.044). The other 2 systematic reviews [21, 22] reported a significant decrease in pain associated with metronidazole use. One review [21] performed a meta-analysis on days 1, 7, and 14; the other review [22] assessed pain on days 1, 2, and 7. Both reviews found a significant reduction in pain at each time point. Both reviews, however, included both oral and topical metronidazole as well as mixed haemorrhoidectomy techniques. We limited our study to oral metronidazole, as topical applications are not commonly used.
Metronidazole resistance may develop. Several studies have shown that nitroimidazole reductase (nim) genes may be found in approximately 2% of Bacteroides fragilis [23-25]. It appears that a range of mechanisms may confer resistance [23], but none as well established or widespread as the more familiar mobile β-lactam resistance genes of the Enterobacteriaceae have emerged. However, metronidazole resistance levels overall remain relatively low. However, antibiotic resistance is approaching epidemic proportions and unnecessary use of antibiotics should be avoided.
There are several limitations to our review. We found on pooled analysis that, despite a significant reduction in VAS across all time points (MD, -1.12; 95% CI, -1.4 to -0.83; P < 0.001), there was significant heterogeneity (I2 = 90%, P < 0.001). On further review, this can be explained by variability in pain assessment; all 4 studies differed in regards to the time points at which pain (VAS) was assessed. This heterogeneity resolved at most time points on subanalysis. Other limitations include the small number of total study participants, and limited duration and follow-up (no study assessed pain beyond 7 days posthaemorrhoidectomy).
While our systematic review reported a reduction in pain associated with metronidazole use posthaemorrhoidectomy, this review has shown that there are limited studies on this topic with only 4 RCTs in the current literature (n = 336). Additional RCTs need to be performed to further delineate the role of oral metronidazole after open haemorrhoidectomy.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.