A Review of the Role of Carcinoembryonic Antigen in Clinical Practice
Article information
Abstract
Carcinoembryonic antigen (CEA) is not normally produced in significant quantities after birth but is elevated in colorectal cancer. The aim of this review was to define the current role of CEA and how best to investigate patients with elevated CEA levels. A systematic review of CEA was performed, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Studies were identified from PubMed, Cochrane library, and controlled trials registers. We identified 2,712 papers of which 34 were relevant. Analysis of these papers found higher preoperative CEA levels were associated with advanced or metastatic disease and thus poorer prognosis. Postoperatively, failure of CEA to return to normal was found to be indicative of residual or recurrent disease. However, measurement of CEA levels alone was not sufficient to improve survival rates. Two algorithms are proposed to guide investigation of patients with elevated CEA: one for patients with elevated CEA after CRC resection, and another for patients with de novo elevated CEA. CEA measurement has an important role in the investigation, management and follow-up of patients with colorectal cancer.
INTRODUCTION
Carcinoembryonic antigen (CEA) was first isolated from human colorectal cancer (CRC) tissue in 1965 by Gold and Freedman [1,2]. It is a foetal glycoprotein and is not usually produced in significant quantity after birth. CEA can become elevated in a number of pathologies. The most common clinical use is surveillance for recurrence of CRC. CEA levels are also sometimes measured in patients without a history of CRC. Elevated CEA in both situations presents a management dilemma to the colorectal surgeon, particularly in deciding the appropriate tests and the subsequent follow-up if those tests are inconclusive, and this led us to conduct this review. This review aims to evaluate the role of CEA in clinical colorectal practice, including the prognostic significance of CEA in patients with CRC, the role of CEA in follow-up after CRC resection, and the management of patients with raised CEA and no history of CRC.
METHODS
A systematic search was performed in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The MeSH headings “Carcinoembryonic antigen,” “CEA,” and “Colorectal Neoplasm” were used to identify relevant studies in the PubMed and Cochrane Library Databases.
Studies included had to be published in English from January 1, 1990, to April 30, 2017, be full text articles, and have comparison groups in which the CEA level was used to determine a component of management or outcome. Data were extracted from the included articles by 2 authors according to the aims of our review: prognostic significance of CEA in patients with CRC, role of CEA in follow-up after CRC resection, and management of patients with raised CEA and no history of CRC. Results were described qualitatively. No statistical analyses were performed.
RESULTS
Of the 2,712 papers identified, 271 were screened in abstract form by 2 authors independently. Thirty-two were deemed relevant, after which the full texts of the articles were obtained and reviewed by 2 authors (Fig. 1). A hand search of the reference lists found no additional studies.
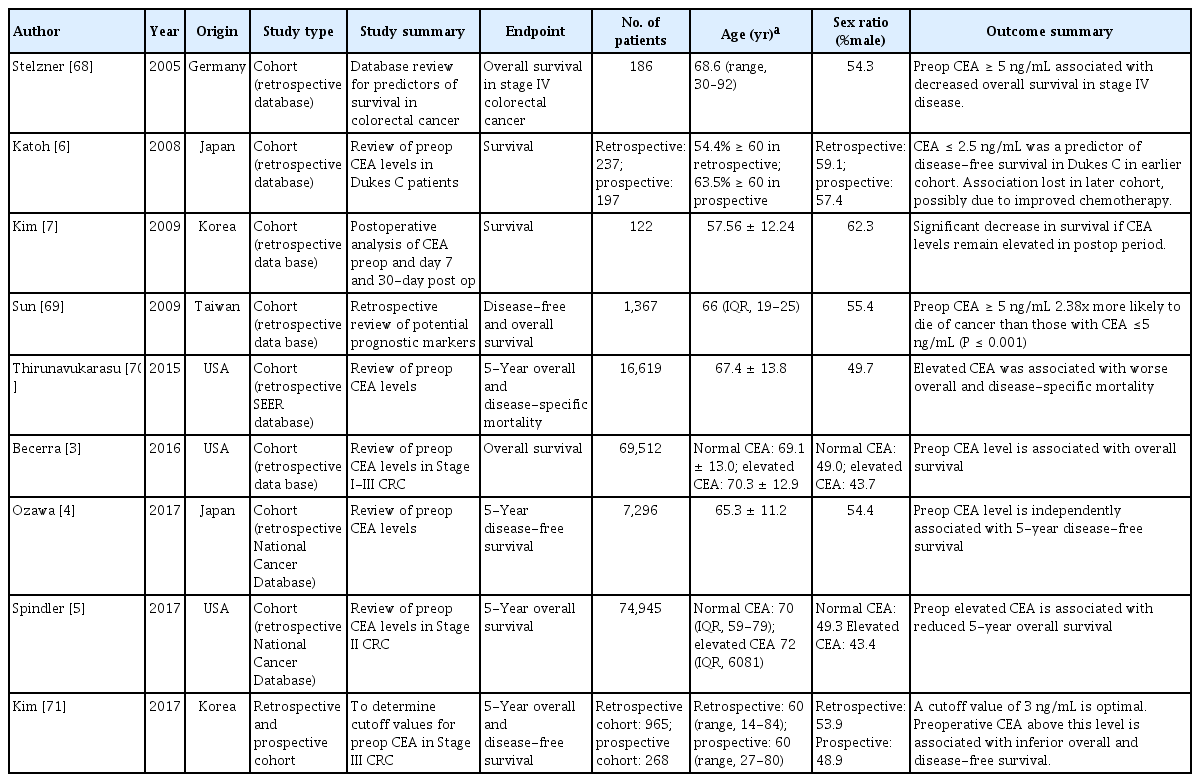
Preoperative CEA and prognosis
Most trials were retrospective (Table 1). Elevated preoperative CEA predicted overall survival across stage I to stage III CRC, with elevated preoperative CEA carrying a 62% increase in the danger of death compared with a normal CEA level [3]. The 5-year disease-free survival was 84.6% vs. 69.8% [4], and the 5-year overall survival was 74.5% vs. 63.4% [5]. However, a study of 2 cohorts with Duke C cancer, 1990–2000 and 2001–2004, found that the predictive value of preoperative CEA was lost in the more recent cohort [6], and may be due to improved chemotherapy regimens. Higher preoperative CEA levels were associated with advanced disease stage.
Postoperative CEA and prognosis
Kim et al. [7] hypothesized that after curative surgery, and therefore after resection of the source of CEA, CEA levels would decrease exponentially. CEA was measured preoperatively and at day 7 and day 30 postoperatively. In the patient group where the CEA level had decreased exponentially, survival was significantly greater than it was in the group where CEA remained elevated, with a trend to increased disease-free survival. Similarly, in patients with stage 4 CRC who underwent a R0 resection, elevated postoperative CEA and CA 19-9 were associated with reduced disease-free survival [8]. Failure of the CEA to return to normal after surgery was indicative of residual or recurrent disease, with CEAs over 10 ng/mL being strongly associated with metastatic disease.
Preoperative CEA and response to neoadjuvant treatment
In rectal cancer, elevated pretreatment CEA levels are associated with a poor response to neoadjuvant treatment, with a retrospective analysis of patients with stage I–III rectal cancer showing significantly decreased pathological complete response, pathological tumor regression, tumor downstaging, and overall survival [9].
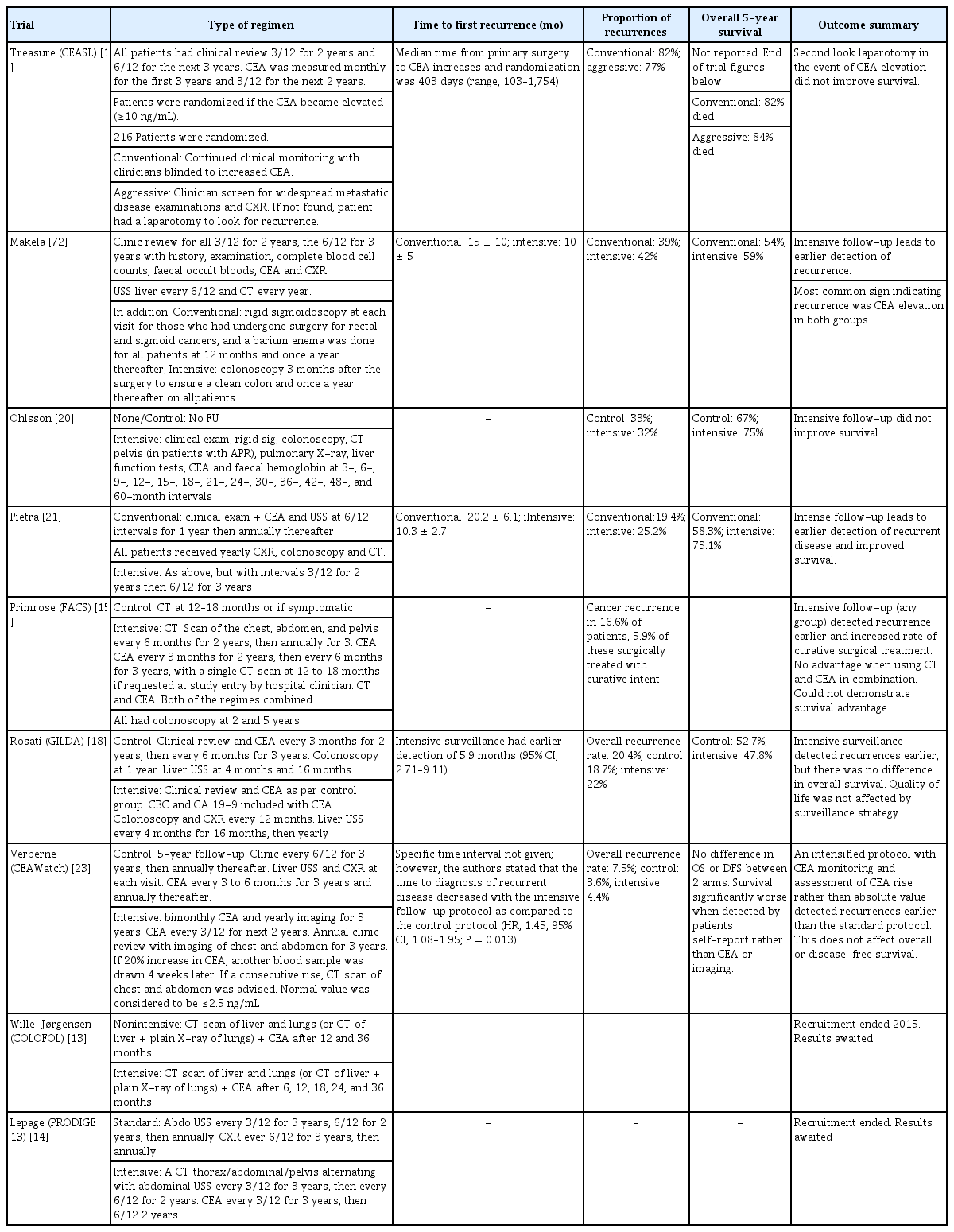
CEA in follow-up after CRC resection
Two recent meta-analyses have shown no survival benefit with increased surveillance after resection for CRC [10, 11]. For CEA specifically, we identified 8 randomized controlled trials (RCT, Table 2). In a RCT looking at managing a rising CEA with a second-look laparotomy compared to conservative treatment [12], no survival advantage was noted, and the study was terminated. CEA began increasing 323 days prior to the onset of clinically evident recurrent disease. A subgroup analysis was not performed, so whether the R0 resection of recurrent or metastatic disease had improved disease-specific survival compared to those with unresectable disease was not clear. The results were also difficult to interpret in the modern setting, as today, a ‘second look’ laparotomy would only be performed on patients with proven resectable disease, and this selected group would be expected to have an improved survival if compared to patients with widespread disease.
More recent trials [13-21] compared a standard follow-up regimen to a higher intensity follow-up, though the regimens were heterogeneous (Table 3). In the FACs (Follow-up After Colorectal Surgery) trial [15], more intensive follow-up with CEA and computed tomography (CT) scanning increased the rate of surgical treatment for recurrent disease, but without survival advantage. The accuracy of a single CEA measurement was poor, with the conclusion that the CEA trend, rather than stand-alone measurements, should be used [22].
The Dutch trial ‘CEAwatch’ investigated the effect of the intensity of the CEA measurement on the detection of recurrent disease and the proportion of curable recurrences [16]. Here, an elevated CEA would trigger repeat CEA testing and, if maintained, chest/abdomen CT. Intensive surveillance (8 weekly CEA) detected more patients with recurrence who had undergone a curative treatment, defined as an R0 resection, compared to the control group (3 monthly CEA: 35% vs. 22%) [16]. No improvement in overall or disease-free survival was found. The only survival benefit was when recurrences were detected by using CEA or imaging as compared to those detected by using patients’ self-reported symptoms [23]. Intensive CEA testing was also found to be cost effective [17]. The GILDA (Gruppo Italiano di Lavoro per la Diagnosi Anticipata) trial investigated intensive surveillance by imaging for patients with Dukes B2-C CRC [18]. In the intensive surveillance arm, recurrences were detected 5.9 months earlier, but no difference in overall survival was reported.
The threshold of serum CEA used to trigger further investigations is controversial and has been addressed by a Cochrane review including 52 studies [24]. Sensitivity and specificity were 82% and 80% for a 2.5 ng/mL cutoff, 71% and 88% for 5 ng/mL, and 68% and 97% for 10 ng/mL, respectively. The review concluded that 10 ng/mL should be used as the threshold to trigger further investigations. Furthermore, the diagnostic accuracy of the postoperative CEA level in detecting recurrence is affected by the preoperative value. With a threshold of 5 ng/mL at 6 months, in patients with curatively resected colon cancer and normal preoperative CEA, the diagnostic accuracy for recurrence was 89.1%, in contrast to patients with an elevated preoperative CEA in whom the accuracy was 58.4%; an increased threshold of > 8 ng/mL in this latter group improved diagnostic accuracy to 75.6% [25].
The hypothesis that CEA level reflects tumor burden in patients with metastatic disease has also been studied by assessing treatment response to chemotherapy. Huang et al. [26] retrospectively analysed 447 patients with metastatic CRC who had undergone resection of the primary tumor and adjuvant chemotherapy. The ratio of posttreatment (after 6 courses of chemotherapy) to pretreatment CEA was strongly correlated with the radiological response and with overall survival [26]. Similarly, in patients with metastatic CRC receiving first-line chemotherapy, there were distinct CEA level slopes according to type of radiological response i.e., progression, partial response, and stable disease [27], suggesting that CEA levels and ratios have a role in the early assessment of treatment response in stage IV CRC. In the FIRE-3 trial, the CEA trend reflected response to targeted therapy [28] in patients with (K)RAS wild-type metastatic CRC. First-line FOLFIRI plus cetuximab gave better overall survival than FOLFIRI plus bevacizumab (28.7 months vs. 25.0 months) [29], with a corresponding faster and greater decrease in CEA level. In the cetuximab arm, CEA responders, defined as a decrease of at least 75%, had better disease-free and overall survivals than CEA nonresponders.
At the cellular level, a correlation between the immunohistochemical status of CEA staining and the serum CEA level was found in colorectal metastases, but not in the primary tumors [30], suggesting that serum CEA levels are influenced both by the production of CEA in tumor cells and by its release into the bloodstream. Positive staining for CEA in metastatic tissue also carried a worse survival. Overexpression of CEA has been reported to inhibit Natural Killer cells and cytotoxic lymphocytes, which may dampen the immune response to metastases [31].
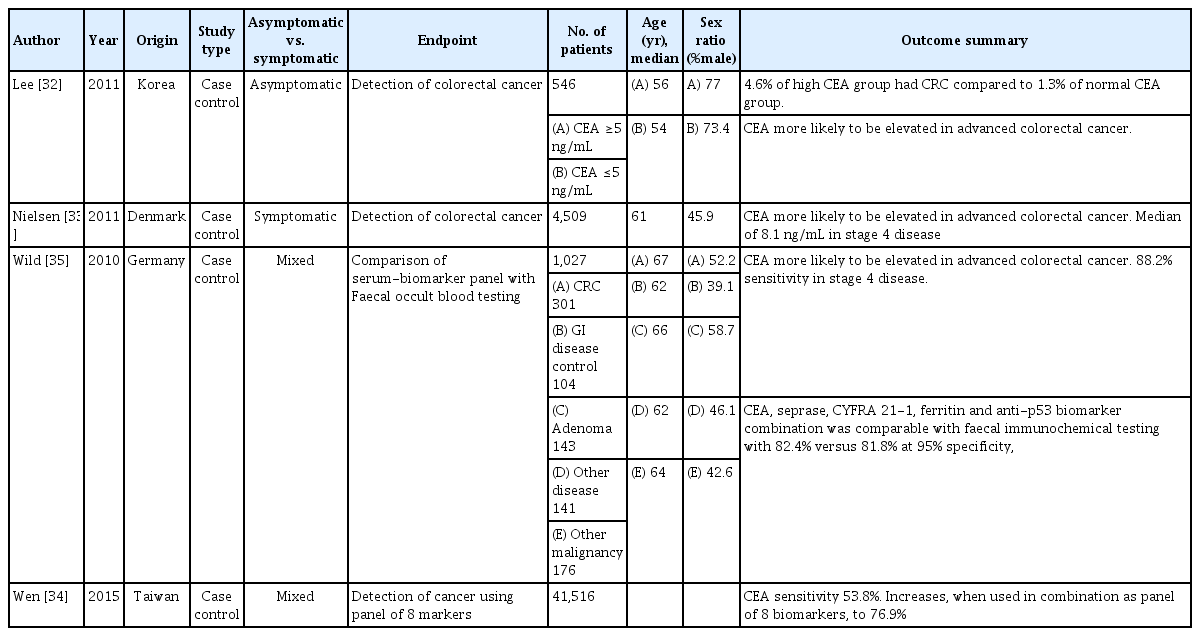
What to do for a patient with a raised CEA and no history of CRC
Whilst no specific trials have addressed what to do for a patient with a raised CEA and no history of CRC, studies have evaluated CEA in screening and diagnosis (Table 4). In patients with elevated CEA, the chance of finding CRC is increased (4.6% vs. 1.3%) [32]. CEA is also a predictor of other diseases, including other cancers, diabetes, chronic lung disease and liver disease [33]. A combination of biomarkers, including CEA, CA 19-9, prostate-specific antigen, and CA125, increases the sensitivity of CEA as a screening modality [34, 35]. In a patient with raised CEA, a detailed history and clinical assessment are likely to be the most important first steps. No evidence was found to support CEA being used as a screening tool.
DISCUSSION
The available evidence supports the use of CEA in surveillance of CRC, particularly in postoperative follow-up. However, no evidence supports its use in screening and diagnosis of CRC, as CEA may be elevated in patients with other pathologies [2, 36-38] and many CRC patients will have a normal CEA. Serum levels > 3-5 ng/mL are deemed to be elevated [39, 40]. Higher baseline levels occur in males, smokers and the elderly [39, 40]. Elevated CEA levels have been associated with a number of benign and malignant conditions (Table 5). CEA is used most frequently in CRC, but it is also a tumor marker in mucinous adenocarcinomas of the endocervix and ovary, as well as in keratinising squamous cell carcinomas of the cervix [41]. CEA levels >10 ng/mL or trending upwards are more commonly associated with malignant conditions [40-43]. Levels > 20 ng/mL are suspicious for metastatic disease [42, 44-46]. As CEA is primarily metabolized in the liver, hepatic dysfunction and biliary obstruction can be associated with raised CEA levels.
In view of the high first-pass hepatic metabolism of CEA (≥90%), very high CEA levels tend to be due to CEA-producing tumors or metastases outside the portal venous drainage territory or to locally advanced tumors within the portal venous system’s drainage territory. Tumor differentiation also affects CEA level [45-47], with 80% of well-differentiated CRCs producing CEA and only 60% of poorly-differentiated tumors produce it [48], making surveillance less reliable. Following CRC resection in patients with normal hepatic function, a 95% reduction to the steadstate postoperative CEA level takes five half-lives, i.e., 35 days [39, 44, 47].
CEA levels correlate with prognosis [49-51]. In CRC, elevated preoperative CEA level (>5 ng/mL) is associated with a higher recurrence rate and disease-related mortality [50-54]. A postoperative CEA decrease is also a prognostic indicator [55, 56], predicting improved overall survival and disease-free survival [28]. This also applies to those having liver surgery for colorectal metastases [57]. CEA level has also been correlated with the presence of circulating cancer cells [54].
The role of pretreatment CEA level in predicting response to chemotherapy or radiotherapy is uncertain, with some studies showing no correlation [58] and with others showing that, compared to CEA <3 ng/mL, an elevated pretreatment CEA (>9 ng/mL) was associated with a poor response to long-course chemoradiotherapy [59]. Reduction of CEA following neoadjuvant chemoradiotherapy for rectal cancer has prognostic significance [60], with CEA ≤5 ng/mL being correlated with increased complete clinical and pathological response and better overall and disease-free survivals. High posttreatment CEA levels may, therefore, identify patients for adjuvant treatment and intensive surveillance, and may perhaps be a relative contraindication to ‘watch and wait’ in patients with apparently complete clinical response.
Surveillance recommendations for CEA in CRC patients vary. The American Society of Clinical Oncology (ASCO) recommends preoperative CEA and postoperative CEA every 3–6 months for at least 5 years [61, 62]. The UK National Institute of Clinical Excellence recommends CEA levels be measured every 6 months for at least 3 years [63]. In addition, ASCO [61] recommends CEA for monitoring the response of metastatic disease to systemic therapy. The CEA doubling time is affected by rate of growth of metastases and by site of recurrence [64, 65].
CRC surveillance: management of patients with elevated CEA levels
A patient with an elevated or progressively rising CEA level with previous CRC should be investigated for recurrent disease (Fig. 2), initially with cross-sectional imaging (CT scan or positron emission tomography-CT [PET-CT]) of the chest, abdomen and pelvis, and not colonoscopy because of CEA’s first-pass metabolism. Both the British Royal Society of Radiology [66] and the US oncology guidelines [67] suggest only performing a PET-CT when other imaging has been normal. If these investigations are negative, a repeat CEA level with clinical review at 3 months is suggested. If the CEA level remains stable without clinical evidence of recurrence, continued CEA measurements every 3 months and clinical review are encouraged. If the CEA level is increasing or >10 ng/mL or if clinical evidence of recurrence is found, repeat cross-sectional imaging is suggested.
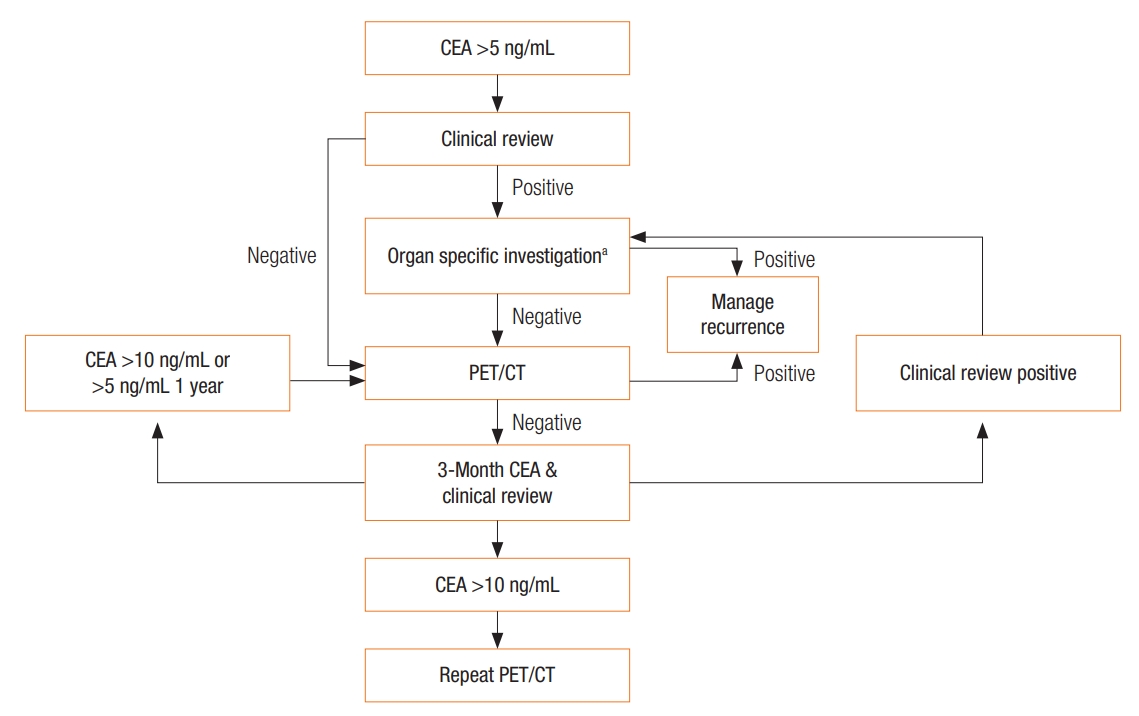
Algorithm 1: Investigation pathway for a patient with a raised carcinoembryonic antigen (CEA) with a previous history of a CEA-expressing cancer. aOrgan-specific investigations: tumor markers, CT scan, colonoscopy, gastroscopy, mammography, cystoscopy, Ultrasound Scan (US), bone scan, biopsy, other test as required. PET/CT, positron emission tomography-computed tomography.
Patients referred with de novo elevated CEA
While CEA should not be used as a diagnostic tool, it often is by the overexuberant health professional. This then raises the dilemma of determining if the elevated CEA is significant, in the absence of a previous history of CRC (Fig. 3). If the patient has a history of previous CEA-producing malignancy (Table 5), the patient should be evaluated in accordance with that particular malignancy.
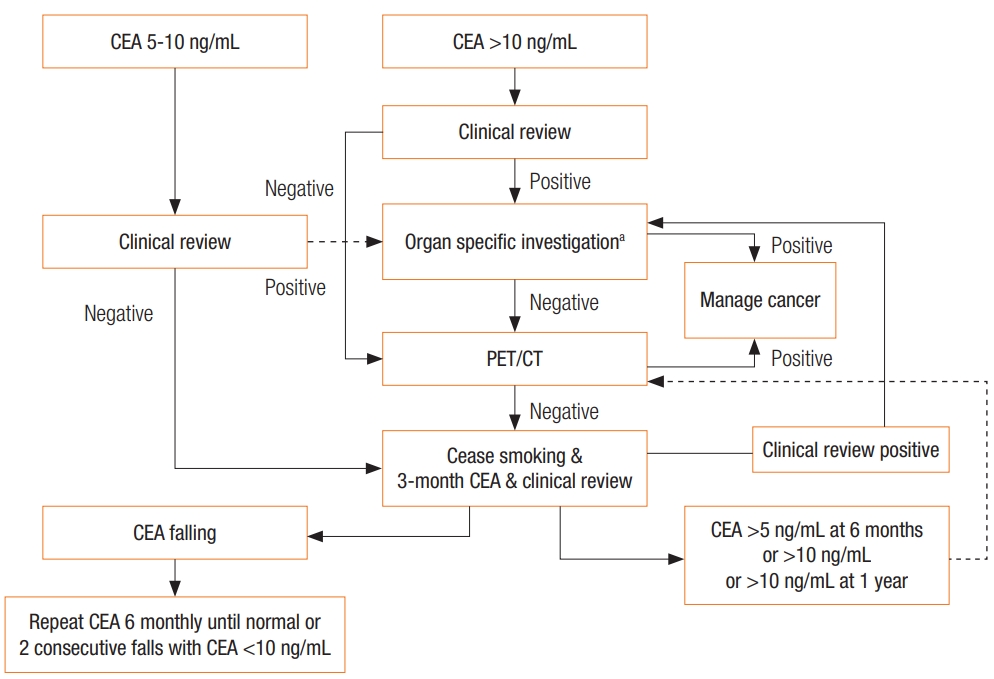
Algorithm 2: Investigation pathway for a patient with a de novo raised carcinoembryonic antigen (CEA). aOrgan-specific investigations: tumor markers, CT scan, colonoscopy, gastroscopy, mammography, cystoscopy, US, bone scan, biopsy, other test as required. Clinical review includes: a full history and examination of the thyroid, breast, thorax, abdomen and pelvis, visual field testing, fundoscopy, long bones examination. Look for melanoma. In females, cervical examination. In males, a prostate examination. PET/CT, positron emission tomography-computed tomography.
In the absence of a history of a previous CEA-producing tumor, evaluation begins with a thorough history and clinical examination, looking for relevant symptoms and signs of CEA-producing benign and malignant conditions (Table 5), and smoking history. Further investigations would include repeat CEA, full blood count, and iron studies, liver and renal function tests, determinations of the CA 125 and calcitonin levels, and so on, as indicated from the clinical review.
If the clinical review does not raise any suspicion of a particular disease process and CEA is <10 ng/mL, no further investigations are recommended at this stage. The patient should be advised to stop smoking for cardiovascular and respiratory health. Determination of the CEA level and a clinical review should be repeated at 3 months. If the level falls, determination of the CEA level and a clinical review should be repeated at 6-month intervals until the CEA level returns to normal or until 2 consecutive decreases are noted. If, however, after 3–6 intervals, the CEA level remains above 5 ng/mL or if the level exceeds 10 ng/mL at any stage, further investigations (CT scan, PET-CT or organ-specific investigations) are indicated.
If the CEA level is greater than 10 ng/mL at the time of presentation, a PET-CT or whole-body CT can be used to look for primary and/or secondary malignancy, unless clinical review indicates a likely site of malignancy to guide a more specific investigations Depending on access and local costs, using a PET-CT scan before organ-specific testing might be more cost effective. If these preliminary tests are negative, a repeat CEA should be performed 3 months later with a clinical review. The clinical review is more important at this stage. A CEA level persistently >10 ng/mL at 1 year requires repeat investigation. Rising CEA levels >10 ng/mL or levels > 20 ng/mL require investigation every year until the underlying cause is detected.
In the clinical scenario of patients with de novo elevated CEA, our practice would be to investigate with whole-body PET-CT before organ-specific investigations, unless clinical evidence suggesting a specific site for the malignancy is found. If PET-CT scanning is not available, we suggest whole-body CT.
CONCLUSION
Current evidence suggests CEA has a role in the prognostication and treatment planning for and the surveillance of patients with CRC. The use of algorithms will help guide how patients presenting with elevated CEA should be further elevated.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.