Oncologic Outcomes of Stage IIIA Colon Cancer for Different Chemotherapeutic Regimens
Article information
Abstract
Purpose
Adjuvant chemotherapy is currently recommended for Stage IIIA colon cancers. This study aimed to elucidate the oncologic outcomes of Stage IIIA colon cancer according to the chemotherapeutic regimen based on a retrospective review.
Methods
From 1995 to 2008, Stage IIIA colon cancer patients were identified from a prospectively maintained database at a single institution. Exclusion criteria were as follows: rectal cancer, another malignancy other than colon cancer, no adjuvant chemotherapy and unknown chemotherapeutic regimen. One hundred thirty-one patients were enrolled in the study, and the clinicopathologic and the oncologic characteristics were analyzed. The number of males was 72, and the number of females was 59; the mean age was 59.5 years (range, 25 to 76 years), and the median follow-up period was 33 months (range, 2 to 127 months).
Results
Of the 131 patients, fluorouracil/leucovorin (FL)/capecitabine chemotherapy was performed in 109 patients, and FOLFOX chemotherapy was performed in 22 patients. When the patients who received FL/capecitabine chemotherapy and the patients who received FOLFOX chemotherapy were compared, there was no significant difference in the clinicopathologic factors between the two groups. The 5-year overall survival and the 5-year disease-free survival were 97.2% and 94.5% in the FL/capecitabine patient group and 95.5% and 90.9% in the FOLFOX patient group, respectively, and no statistically significant differences were noted between the two groups.
Conclusion
Stage IIIA colon cancer showed good oncologic outcomes, and the chemotherapeutic regimen did not seem to affect the oncologic outcome.
INTRODUCTION
Colorectal cancer is one of the leading causes of death in the Western world. According to 2011 cancer statistics in Korea, colorectal cancer is the second most common type of cancer in males and the third most common type of cancer in females [1].
Although the most important modality in the treatment of colon cancer is the curative resection, adjuvant chemotherapy following curative resection has reduced the recurrence and mortality in patients with stage III colon cancer [2]. Until the early 1990's, 5-fluorouracil (5-FU) was the single effective chemotherapy available, but only led to meaningful responses in a small minority of treated patients. The recently-introduced combination of oxaliplatin and infusional 5-FU/leucovorin (FL) significantly increased the response rate and the time to progression compared with 5-FL regimen [3, 4]. These results established combination therapy of 5-FU and oxaliplatin as the standard first-line chemotherapy regimen for patients with stage III colon cancer. Capecitabine is an oral form designed to mimic continuous infusion of 5-FU. Capecitabine is considered to be as effective and more tolerable than 5-FU [5]. Furthermore, in some studies, capecitabine has been shown to exhibit antitumor activity similar to that of 5-FL [6]. In the National Comprehensive Cancer Network (NCCN) 2012 guidelines, adjuvant chemotherapy with a combination of oxaliplatin and infusional 5-FL (FOLFOX) was recommended for patients with stage III colon cancer, and FL or capecitabine was recommended as another option.
In the 7th American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging system, Stage IIIA colon cancer exists when the depth of the tumor is limited to the muscularis propria and the number of metastatic lymph nodes is less than three, or when the tumor has invaded the submucosa and the number of metastatic lymph nodes is four to six. The observed survival rates for 28,491 patients with an adenocarcinoma of the colon were as follows: The 5-year survival rate for stage I, stage IIA, stage IIB, stage IIC, Stage IIIA, stage IIIB, stage IIIC, and stage 4 colon cancer were 74.0%, 66.5%, 58.6%, 37.3%, 73.1%, 46.3%, 28.0%, and 5.7%, respectively. In the above data, the 5-year survival rate for Stage IIIA colon cancer was similar to the 5-year survival rate for stage I colon cancer or stage IIA colon cancer [7-9]. In addition, patients with Stage IIIA colon cancer are frequently diagnosed with stage I colon cancer preoperatively because of the low detection rate of lymph-node involvement with preoperative imaging.
Because of the good outcomes for Stage IIIA colon cancer, some clinicians may not be sure whether to offer postoperative chemotherapy, which potentially has an impact on morbidity and quality of life. Although other data support the role of postoperative chemotherapy in the treatment of stage III colon cancer, data regarding the role of postoperative chemotherapy in only Stage IIIA colon cancer are limited. This study aimed to elucidate the oncologic outcomes of Stage IIIA colon cancer according to the chemotherapeutic regimen.
METHODS
From 1995 to 2008, patients with Stage IIIA colon cancer were identified from the prospectively maintained colorectal cancer database at Samsung Medical Center. The database contained detailed information on patient characteristics, operative findings, histology, laboratory findings, and adjuvant therapies. Follow-up survival data were collected retrospectively through medical-record analyses and phone interviews.
Patients with Stage IIIA colon cancer who underwent a curative colonic resection were included in the study. Exclusion criteria were as follows: rectal cancer, another malignancy other than colon cancer, patients who did not receive adjuvant chemotherapy and patients who did not have any information about the chemotherapeutic agents. Cancer was staged by using the AJCC 7th TNM staging system. One hundred fifty-four patients who underwent surgery between 1995 and 2008 were categorized as having Stage IIIA colon cancer. Among the 154 patients, 23 patients were excluded: 8 patients in whom adjuvant chemotherapy was not performed, 6 patients with rectal cancer, 6 patients having synchronous malignancy, and 3 patients who did not have the details of chemotherapeutic regimens. Finally, 131 patients were enrolled for analysis in the present study. The mean age of the enrolled patients was 59.5 years (range, 25 to 76 years), the number of males was 72 and the number of females was 59, and the median follow-up period was 33 months (range, 2 to 127 months).
Chemotherapeutic regimens for Stage IIIA colon cancer included FL, capecitabine and FOLFOX. The FL regimen was performed as one cycle of FU 500 mg/m2 (bovine serum albumin) and leucovorin 20 mg/m2 intravenously for 2 hours daily for 5 days, followed by a 3-week rest period. Six cycles of the FL regimen were performed until disease progression ceased, intolerable toxicity developed or the patient refused further chemotherapy. Capecitabine was administered at a dose of 1,250 mg/m2 twice a day for 14 days; then, it was not administered for the next 7 days. This three-week period was considered as one cycle, and a total of 8 cycles was considered as the standard of care. FOLFOX chemotherapy was performed according to the modified FOLFOX6 regimen. The modified FOLFOX6 regimen is comprised of intravenous infusion of oxaliplatin, 85 mg/m2, plus leucovorin, 400 mg/m2, over two hours, FU, 400 mg/m2, over 5 minutes, and then slow intravenous infusion of fluorouracil, 2,400 mg/m2, over 46 hours. The cycle was repeated every 2 weeks for 12 cycles.
The patients were scheduled for follow-up visits every 3 to 4 months for the first two years, every 6 months for the next 3 years, and then annually thereafter. For most of the patients, their follow-up assessment at each visit included physical examination, abdominal and pelvic computed tomography (CT) scans, and chest X-rays. Colonoscopies were performed during the first, third, and fifth year of follow-up. Abnormal physical findings or laboratory results led to further screening using ultrasonography, abdominal and pelvic CT scans, chest CT scans, magnetic resonance imaging, or positron emission tomography CT as per the clinician's decision.
SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA) was used as the statistical analysis program. The chi-square test was used for analyzing the data, and the Kaplan-Meier method was used to analyze the overall and the disease-free survival rates. The results were considered as statistically significant when the P-value was less than 0.05.
RESULTS
Patient demographics and tumor characteristics
Among the 131 patients, 23 patients (17.5%) had lesions in their right colon, and the other 108 patients (82.5%) had lesions in their left colon. Forty-five patients (34.4%) had tumors invading the submucosa, and 86 patients (65.6%) had tumors invading the muscularis propria. Eighty-four patients (64.1%) had a single metastatic lymph node, and 47 patients (35.8%) had multiple metastatic lymph nodes. The Stage IIIA tumor were sub-staged as T1N1a in 30 patients (22.9%), T1N1b in 16 (12.2%), T2N1a in 54 (41.2%), T2N1b in 31 (23.6%), and T1N2a in 0 patients (0%).
Regarding chemotherapeutic regimens, FL chemotherapy was performed in 43 patients (32.8%), capecitabine in 66 patients (50.3%), and FOLFOX in 22 patients (16.7%). When the patients were categorized into two groups, the FL/capecitabine patient group and the FOLFOX patient group, the numbers of patients in the two groups were 109 (83.2%) and 22 patients (16.7%), respectively. There were no differences in the clinicopathologic factors in terms of depth of invasion and nodal status between the two groups (Table 1). The proportion of right-sided colon cancer was higher in the FOLFOX patient group; however, that difference did not reach statistical significance (P = 0.053).
Survival and recurrence
During the follow-up period, recurrence occurred in 8 patients (6.1%). The patterns of recurrence were liver metastasis in four patients, peritoneal dissemination in two patients, pancreatic metastasis in one patient, and multiple sites of recurrence in one patient. Among them, four patients died from cancer progression during the follow-up period. All mortality cases were related to cancer progression. The estimated five-year overall survival rate was 96.9%, and the estimated five-year disease-free survival rate was 93.9%.
When comparing the FL/capecitabine and FOLFOX patient groups, recurrences occurred in six patients (4.5%) of the FL/capecitabine group, and among them, three patients died during the follow-up period. The patterns of recurrence were hepatic metastases in three patients, pancreatic metastasis in one patient, peritoneal dissemination in one patient, and multiple sites of metastasis in one patient. In the FOLFOX patient group, two patients (9.0%) had recurrence, and only one patient died. The recurrence patterns were liver metastasis in one patient and intraperitoneal metastasis in one patient. There was no statistically significant difference in the recurrence rates between the two groups (P = 0.241).
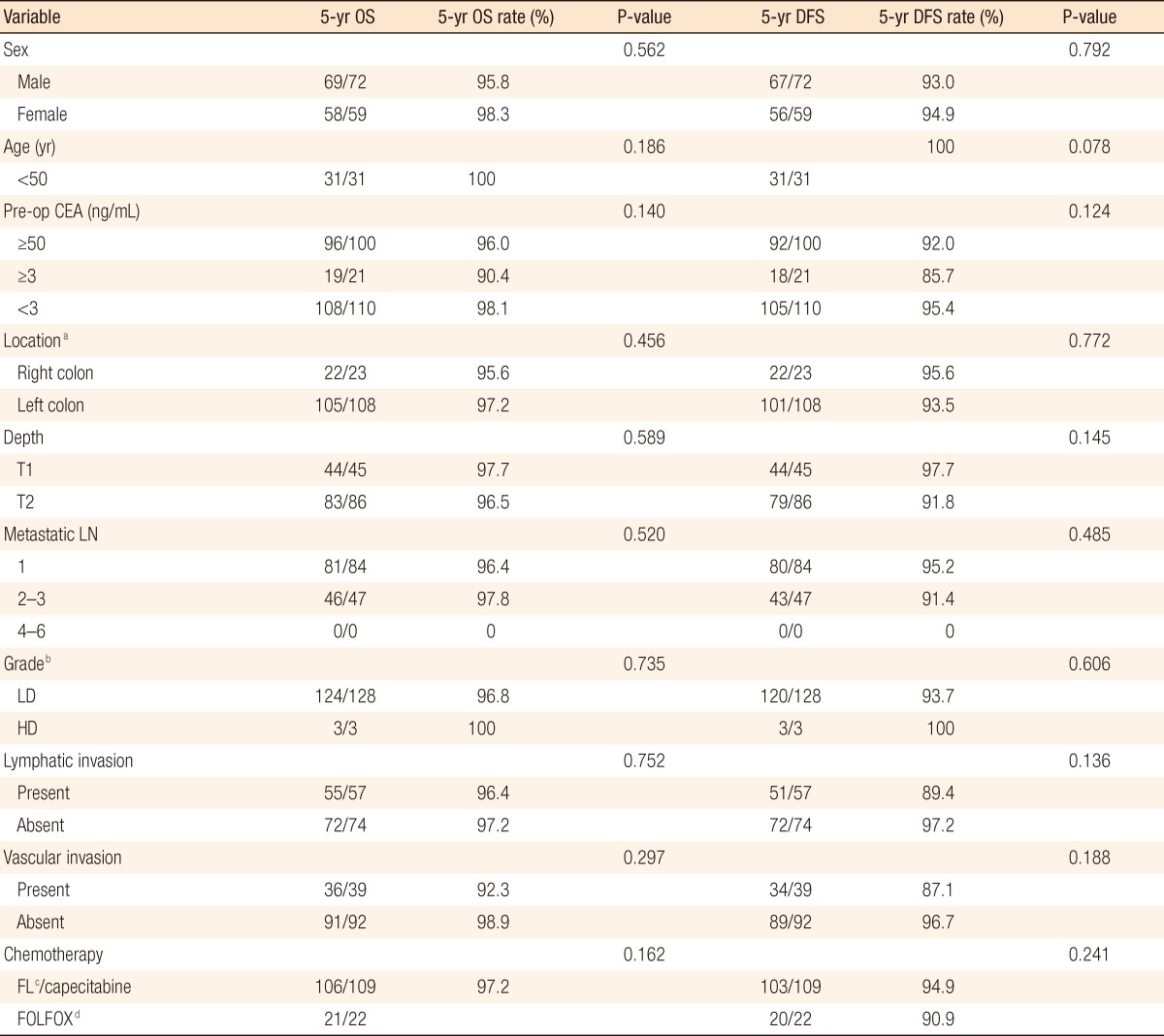
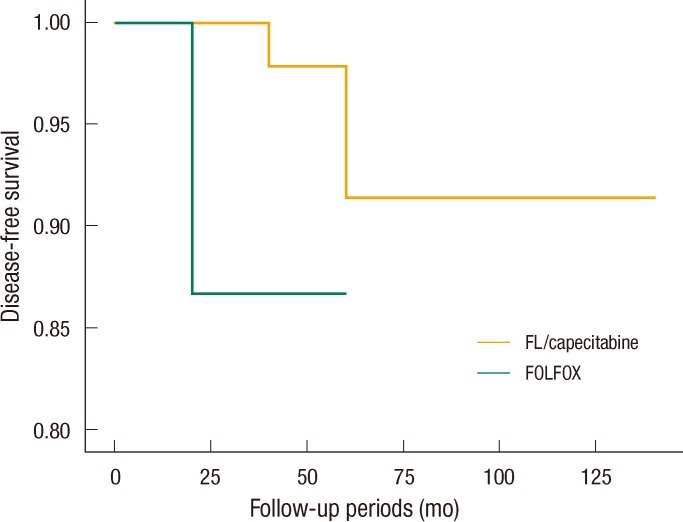
The estimated 5-year overall survival rates were 97.2% in the FL/capecitabine patient group and 95.5% in the FOLFOX patient group, but that difference in the estimated 5-year overall survival rates between the two groups was not statistically significant (P = 0.162) (Fig. 1). The estimated 5-year disease-free survival rates in the FL/capecitabine patient group and the FOLFOX patient group were 94.5% and 90.9%, but that difference in the estimated 5-year overall survival rates between the two groups was not statistically significant (P = 0.241) (Table 2, Fig. 2). In the multivariate analysis, survival rates were not related to any factors, including depth of tumor invasion, the number of metastatic lymph nodes, lymphovascular invasion and chemotherapeutic regimen.
Estimated five-year overall survival rates of each chemotherapeutic regimen. FL, fluorouracil/leucovorin.
DISCUSSION
Successful treatment of colon cancer requires a multidisciplinary approach, frequently combining surgery and chemotherapy. Postoperative adjuvant chemotherapy was not common prior to 1990. Based on the results of many clinical trials, adjuvant chemotherapy has been proposed to reduce the incidence of recurrence of colon cancer and to improve survival, and it was included in the standard therapy for stage III colon cancer [10, 11].
Until the 1990's, the FL regimen was widely applied as the adjuvant therapy. Oxaliplatin with infusional 5-FU, namely FOLFOX, has been reported to be effective and comparatively safe, and is now recommended as the standard first-line chemotherapy for patients with colon cancer [12, 13]. However, FOLFOX chemotherapy has many adverse effects, such as peripheral neuropathy, leukopenia, colitis, and thrombocytopenia; hence, many clinicians have made efforts to prevent and treat the adverse effects of FOLFOX chemotherapy [14-16]. To enhance convenience and reduce adverse effects, oral chemotherapeutic agents like capecitabine have been considered for alternative first-line chemotherapy. Compared to the FL regimen, capecitabine has shown a substantially lower incidence of clinically important toxicities, and similar oncologic outcomes [17].
In this study, we investigated only Stage IIIA colon cancer patients in a single institution. Although many studies of adjuvant chemotherapy for stage II or III colon cancer have been reported [18-20], reports on the oncologic outcomes for Stage IIIA colon cancer according to the chemotherapeutic regimen are rare. The present study analyzed the oncologic outcomes of Stage IIIA colon cancer according to only the chemotherapeutic regimen. Our data showed very good overall and disease-free survival rates, and those rates were much superior to those in other studies [9]. It is quite difficult to identify clear reasons for the excellent clinical outcomes in the present study. The most likely reasons for the good oncologic outcomes may be that the specialists who performed the colorectal cancer surgery in all patients had performed more than one hundred colorectal cancer surgeries every year and that we only included those patients who received adjuvant chemotherapy as per the NCCN guidelines. Some other factors influencing the survival rates, including ethnic differences and dietary patterns, might exist, but they were not evident.
Although we excluded patients who did not receive chemotherapy during the study period, we had eight patients with Stage IIIA colon cancer who did not receive adjuvant chemotherapy for some reason. Among them, three patients had recurrences during the follow-up period; there were two cases of peritoneal dissemination and one case of liver metastasis. Due to the very small number of patients in this group, comparing this group directly with the other groups was difficult. However, although not an original goal of this study, this result emphasizes the need for adjuvant chemotherapy in cases of Stage IIIA colon cancers.
In the present study, the chemotherapeutic regimens were not decided based on any specific guideline, but according to the clinician's preference and the treatment period. In other clinical trials, FOLFOX chemotherapy may have frequently caused troublesome complications, and especially, peripheral neuropathy may have lasted for several years. We found excellent oncologic outcomes of Stage IIIA colon cancer, and we did not find any statistically significant difference between the FL/capecitabine and the FOLFOX regimens. Based on our findings, selection of those regimens, which do not cause severe complications in patients with Stage IIIA colon cancers, should be considered.
The present study has the limitation of retrospective clinical observation. In addition, a bias in the selection of the two groups may have existed even though the two groups shared similar clinical and pathological characteristics, types of surgery. Finally, the sample sizes of the two groups were too small to have enough statistical power. Basically, the principle and the regimens of chemotherapy conformed to the NCCN guidelines. However, selection of each chemotherapeutic agent was individualized by performance, patient's desire, or clinician's preference without any corrected selection criteria. We thought that anticipating the effect of chemotherapy might be another selection bias. Hence, extending our findings to a larger population in order to have a general consensus may be very difficult. However, this study analyzed a relatively large number of a rare subset of colon-cancer patients in a single institution; moreover, all the enrolled patients were followed for more than three years. We believe that our results could provide a small clue for understanding the clinical outcomes of Stage IIIA colon cancer and for designing prospective multicenter trials.
Conclusively, our data suggest that Stage IIIA colon cancer has excellent oncologic outcomes after curative surgery and adjuvant chemotherapy. Also, a need seems to exist to restage the group of Stage IIIA colon cancer patients and to reevaluate the risks and benefits of adjuvant chemotherapeutic regimens, and the selection of chemotherapeutic regimen must be based on the patient's status, with a less toxic therapy possibly being considered, especially in immune-compromised patients with Stage IIIA colon cancer. Future prospective and multi-center studies are needed to confirm this observation.
Notes
No potential conflict of interest relevant to this article was reported.