- Search
Abstract
Total mesorectal excision (TME) has gained worldwide acceptance as a standard surgical technique in the treatment of rectal cancer. Ever since laparoscopic surgery was first applied to TME for rectal cancer, with increasing penetration rates, especially in Asia, an unstable camera platform, the limited mobility of straight laparoscopic instruments, the two-dimensional imaging, and a poor ergonomic position for surgeons have been regarded as limitations. Robotic technology was developed in an attempt to reduce the limitations of laparoscopic surgery. The robotic system has many advantages, including a more ergonomic position, stable camera platform and stereoscopic view, as well as elimination of tremor and subsequent improved dexterity. Current comparison data between robotic and laparoscopic rectal cancer surgery show similar intraoperative results and morbidity, postoperative recovery, and short-term oncologic outcomes. Potential benefits of a robotic system include reduction of surgeon's fatigue during surgery, improved performance and safety for intracorporeal suture, reduction of postoperative complications, sharper and more meticulous dissection, and completion of autonomic nerve preservation techniques. However, the higher cost for a robotic system still remains an obstacle to wide application, and many socioeconomic issues remain to be solved in the future. In addition, we need more concrete evidence regarding the merits for both patients and surgeons, as well as the merits compared to conventional laparoscopic techniques. Therefore, we need large-scale prospective randomized clinical trials to prove the potential benefits of robot TME for the treatment of rectal cancer.
Total mesorectal excision (TME), introduced by Heald et al. [1] in 1982 has revolutionized the surgical management of rectal cancer. TME has gained worldwide acceptance as a standard surgical technique. Actually, its use can result in a dramatic reduction of the local recurrence rate [2]. Its principles have been advocated for sharp pelvic dissection based on pelvic anatomy, subsequently resulting in not only en-bloc removal of rectal cancer and surrounding mesorectum containing a lymph node, but also pelvic autonomic nerve preservation [2]. The quality of the macroscopic specimen for rectal cancer has been regarded as an important parameter for prediction of prognosis [3, 4]. Oncologically, there must be no damage to the mesorectum during dissection, and a clear circumferential resection margin and distal resection margin should be obtained after resection.
With the development of new technologies, laparoscopic surgery has been applied to colorectal cancer resection [5]. Initially, there were many concerns regarding oncologic safety, even though it was associated with better short-term clinical outcomes, such as earlier recovery of bowel function, less pain, cosmetic advantages, shorter hospital stay, etc. [6]. Therefore, a couple of prospective randomized clinical trials were conducted to compare the operative and oncologic safety between open and laparoscopic surgery for the treatment of colorectal cancer [7-10]. As a result, in comparison with open surgery, oncologic safety was confirmed for colon resection by laparoscopy [8]. However, for rectal cancer, the issue of oncologic safety has not yet been adequately resolved. Rather, there are still some concerns regarding laparoroscopic rectal surgery due to the high conversion rate or the high proportion of circumferential resection margin positive rates in the Medical Research Council Conventional versus Laparoscopic-assisted Surgery in Colorectal Cancer (MRC_CLASICC) trial [10]. These disappointing early results may originate from many factors. The inherent limitations of laparoscopic rectal resection, such as an unstable camera platform, limited mobility of straight laparoscopic instruments, two-dimensional imaging, and a poor ergonomic position for surgeons may be some of those factors.
Robotic technology was developed in an attempt to reduce the limitations of laparoscopy [11, 12]. The current system, the da Vinci™ robot (Intuitive Surgical Inc., Sunnyvale, CA, USA), overcomes many of the inherent constraints of laparoscopy, such as the fulcrum effect, poor depth perception, decreased range of motion, and instrument tremor. In addition, robotic technology allows motion scaling, surgeon-controlled three-dimensional camera navigation, and remote telesurgical applications [11]. The robotic system was adapted to rectal cancer surgery with the expectation that secure dissection of an avascular plane between the presacral fascia and the fascia propria of the rectum without injury to the integrity of the mesorectum in the narrow pelvic cavity could be more easily performed by using precise robotic arms and a stable stereoscopic magnified view with high illumination.
However, with respect to the treatment of rectal cancer, what is the best method for both patients and surgeons? The extent and the quality of a surgical resection must be identical, regardless of any modalities. Even though laparoscopy or robotic surgical techniques have shown better short-term clinical outcomes, fundamental concepts and the extent of surgical techniques should not differ from those of open surgery, meaning that any modalities must keep the principles of TME. Therefore, some concerns regarding oncologic safety in minimally invasive rectal cancer surgery still remain.
A multimodality team approach (MTA) according to the patient's initial stage has recently been considered as an essential tool for improving the oncologic outcome and the quality of life of a patient with rectal cancer. MTA means that treatment methods should be discussed and decided by cooperation of various departments, including surgery, radiation oncology, medical oncology, gastroenterology, diagnostic radiology, etc. A proper surgical approach should also be discussed and decided using MTA. Even if surgeons may choose minimally invasive surgery (laparoscopy or robot), the most important consideration is that they should follow the same principles of standard surgical techniques and perform the standard extent of surgery, which are essential for a patient's cure. In this review, we attempt to investigate the current status and potential benefits of minimally invasive surgery, compared with open surgery, in the treatment of rectal cancer, with consideration for multidimensional aspects.
Data retrieved from the Health Insurance Review and Assessment (HIRA, Korea) service by the Korean Study Group of Laparoscopic Colorectal Cancer Surgery shows that the annual number of surgical procedures for the treatment of colorectal cancer in Korea increased year by year and reached 13,683 cases in 2008. Among them, there were 4,762 cases of rectal cancer (Fig. 1).
The penetration rate of laparoscopic surgery for the treatment of colon cancer was 27.8% (3,144/11,325) in 2006, which increased to 49.1% (6,715/13,682) in 2008. The penetration rate of laparoscopic surgery for the treatment of rectal cancer was 24.5% (1,098/4,478) in 2006, which also increased to 48.1% (2,290/4,763) in 2008 (Fig. 2). As far as we know, this penetration rate was as high as that of Japan. Compared to the relatively higher penetration rates in Korea, the average penetration rate of laparoscopic surgery in cases of colorectal cancer was estimated at below 20% in the USA and Europe [13, 14].
The low penetration rate in the USA may have originated from the high prevalence rate of obese patients, which causes greater difficulty and is more time-consuming than open surgery for adequate surgical dissection, such as TME, for the treatment of rectal cancer. Rea et al. [14] reported interesting data at the 2010 American Society of Colon and Rectal Surgeons (ASCRS) meeting (Minneapolis, MN, USA). They analyzed data regarding utilization of a laparoscopic colectomy in the USA before and after the Clinical Outcomes of Surgical Therapy (COST) Study Group Trial. Elective laparoscopic surgery for treatment of benign disease increased from 5.3% in 2002-2003 to 8.3% in 2005-2006 while the laparoscopic colectomy for the treatment of cancer increased by a larger percentage over the same time frame, 2.6% to 7.9%. They concluded that within two years after publication of the COST trial, the use of a laparoscopic resection for the treatment of colon cancer almost equalled that of benign disease. However, over 90% of cases are still performed with an open approach, and this treatment option is influenced by socioeconomic factors, including gender, race, income, health insurance status, and hospital type.
Laparoscopic rectal cancer surgery has some limitations, including a 2-dimensional view, demands for a skilled assistant for a stable camera and proper retraction of the bowel, and limited dexterity of the laparoscopic instruments owing to a narrow pelvic cavity. These various factors result in a steep technical learning curve for laparoscopic TME, which is not easy to overcome [15, 16].
The robotic system has many advantages, including a more ergonomic position, stable camera platform and stereoscopic view, as well as elimination of tremor and subsequent improved dexterity. Due to these advantages of the robotic system, it has been applied to various complex surgeries, including prostatectomies, esophagectomies, gastrectomies, thyroidectomy, pancreaticoduodenectomies, hepatectomies etc. [11]. Thus far, a couple of sets of data have been published in many fields of cancer surgery. Likewise, robot-assisted TME affords the surgeon an improved 3-dimensional vision, ergonomics of the operating console, tremor elimination, superior dexterity, and relief of surgeons' discomfort, which could result in attenuation of the necessary learning curve for minimally invasive mesorectal excision.
So far, two different modalities of robotic rectal cancer surgery have been applied in clinical practice. One is the hybrid method, and the other is the totally robotic surgery. In brief, the hybrid method is composed of a laparoscopic procedure (mobilization of the sigmoid and the left colon, ligation of the inferior mesenteric artery and vein) and a robotic procedure (pelvic dissection). A totally robotic method means that all procedures are performed with a robotic system. Each procedure has its own merits and demerits. We need more data to compare the efficacies of the various methods.
One of the most outstanding features is that the so-called 'Natural Orifice Specimen Extraction (NOSE)' technique can be performed more easily by using a robot. In the NOSE procedure, specimen retrieval is carried out using a natural orifice (e.g., anus or vagina) instead of a minilaparotomy. Choi et al. [17] reported their initial experience of transanal or transvaginal retrieval of the specimen in robotic-assisted anterior resection. They concluded that robotic-assisted laparoscopic methods were safe and feasible modalities to avoid the traditional abdominal incision. Following these results, they showed that the NOSE procedure could be more easily performed using a robotic system than a conventional laparoscopic technique and that it could reduce the postoperative pain score compared to a conventional minilaparotomy [18].
Another advancement is education derived from the progress of technology. As the da Vinci system has been developed, we can now use two console boxes. Because of this system, less-experienced surgeons are more apt to be trained for robotic technique with supervision by a more-experienced surgeon. Although there are many potential advantages in the robotic system for rectal cancer surgery, unfortunately, in terms of both surgeons and patients, there has been a lack of concrete evidence of the benefits.
Since the introduction of the robotic system for use in rectal cancer surgery, the safety and feasibility have been confirmed by several studies [19-22]. A few comparative results for laparoscopic versus robotic TME have recently been published [23-26]. Except for one randomized design, most of those were non-randomized comparative studies. With the current evidence, we can investigate early postoperative morbidity and oncologic aspects regarding specimen quality.
Conversion rates ranged from 0 to 22% for a laparoscopic TME (LTME) and from 0 to 7.3% for a robotic TME (RTME). In one randomized study by Baik et al. [25], the conversion rate for a RTME was found to be statistically lower than that for a LTME (0% vs. 10.5%, P = 0.013). However, in other studies, no difference in conversion rate was observed between the two modalities.
With the exception of one study, the operative time showed no difference. Park et al. [24] reported longer operative times in the robotic group than in the laparoscopic group (231.9 minutes vs. 168.6 minutes, P < 0.001). This result could be explained by the surgeon's previous abundant experience of more than 400 cases of laparoscopic resection before starting robotic rectal resection, as reported in the study by Park et al. [24] There were no differences for parameters reflecting difficulties of dissection, such as median estimated blood loss, hemoglobin change during surgery or need for intraoperative transfusion between LTME and RTME.
No difference in the postoperative complication rate was observed between LTME and RTME, ranging from 19.3% to 26.8% in LTME and from 10.7% to 29.3% in RTME. The most serious complication after rectal resection is anastomotic leakage. The anastomotic leakage rate ranged from 1.7% to 9.7% for RTME. In contrast, it ranged from 2.4% to 7.5% for LTME. No difference was observed between the two modalities. However, Baik et al. [25] reported a low rate of serious complications, including anastomotic leakage, in RTME (5.4% vs. 19.3%, P = 0.025). In their study, there was only one anastomotic leakage for RTME and four anastomotic leakages for LTME.
Bianchi et al. [26] reported one reoperation due to peritonitis in post operative day after RTME. This complication originated from a small bowel perforation. Even though it is rare, surgeons who start robotic surgery should be aware of this kind of complication originating from unfamiliar handling of the robotic arm.
Baik et al. [25] reported on the earlier start of a soft diet and a shorter hospital stay with RTME. However, other studies have reported no difference for the first bowel movement, start of diet, and length of hospital stay.
Use of robotic surgery for the treatment of rectal cancer has been relatively recent; therefore, data regarding long-term oncologic outcome are very limited. Baek et al. [27] recently reported their oncologic outcome as a 96.2% 3-year overall survival and a 73.7% 3-year disease-free survival with a mean follow up of 20.2 months. However, in cases of comparison studies between LTME and RTME, no result on long-term oncologic outcomes has been reported. Instead, most studies have compared the distal resection margin, the circumferential resection margin's involvement status, and the numbers of total harvested lymph nodes as early oncologic parameters. No difference in circumferential resection margin involvement rates has been reported. The median distal resection margin ranged from 2.1 cm to 4.0 cm in RTME and from 2.3 cm to 3.8 cm in LTME. The median numbers of harvested lymph nodes were 13.1 to 18.4 for RTME and 14.2 to 18.7 for LTME. No difference was found with respect to distal resection margin and numbers of harvested lymph node numbers. Of particular interest, Baik et al. [27] compared the quality of the mesorectum after completion of surgery. Macroscopic judgements of the specimen were classified as complete, nearly complete, and incomplete. Superiority of mesorectal integrity in RTME compared to LTME was observed in that study (P = 0.033). However, with respect to early oncologic outcomes, no difference was observed between laparoscopic versus robotic TME, showing oncologic safety for both RTME and LTME. These results suggest that according to the current evidence, there is no difference for postoperative recovery, morbidity, and early oncologic outcomes between RTME and LTME. With respect to conversion rate and operative time, even though in some studies, RTME showed better outcomes, more data are required in order to draw a definite conclusion.
Another point is that, the competence of the surgeon in either modality may affect the surgical outcome. No difference in outcomes was observed between LTME and RTME when LTME was performed by an experienced laparoscopic surgeon. In some points of view, this may be a reflection of the short learning curve of RTME. Therefore, to clarify the real benefits of RTME, surgeon factors should also be considered in future studies.
In the performance of laparoscopic surgery, an assistant should control the laparoscopic image by directing the laparoscopic camera on the operative field, following the instructions of the surgeon. This task requires ongoing active communication between the surgeon and the assistant in the same operation theater. Therefore, physical space conflicts or inevitable confusion between the surgeon and the assistant may arise during surgery. Besides, human camera control may result in a suboptimal image due to tremor, off-center drift, or loss of horizontal orientation; therefore, frequent correction is required during surgery. In addition, inadvertent collisions with tissue may result in the need to clean the lens frequently. These problems tend to diminish the concentration of the surgeon and impede the flow of the operation. However, the robotic system has a self-controllable camera platform, which is very stable and provides a stereoscopic view. For this reason, much attention is now being paid to the robotic surgery.
Van Koughnett et al. [28] developed a subjective scale for assessing robotic surgery. Twenty choledochojejunostomies were performed in an ex-vivo pig model. Ten anastomoses were performed laparoscopically, and ten anastomoses by using da Vinci robot assistance. They completed the scale form after each procedure. Robotic surgery was associated with superior ease, compared to laparoscopy, in 8 of the 13 factors, including image quality, depth perception, comfort, eye fatigue, dexterity, precision of motion, speed of motion, and range of motion.
One possible advantage of robotic-assisted surgery is that surgeons may feel more comfortable using robotic assistance over conventional laparoscopy, which might originate from surgeon's sitting at a console during surgery, the fine Endowrist™ (Intuitive Surgical, Sunnyvale, CA, USA) movement of the da Vinci system, etc. However, little evidence is available to substantiate this potential benefit. Future research must be planned in order to validate the subjective assessment scale for the evaluation of surgeon's fatigue.
Robotic technology was developed in an attempt to reduce many of the limitations of laparoscopy while maintaining its minimally invasive nature. Stefanidis et al. [29] reported the intracorporeal suture to be one of the most difficult laparoscopic tasks. They assessed the impact of robotic assistance on the suturing performance of a novice and on the safety and the workload in the operating room. Results revealed that robotic assistance resulted in significantly improved intracorporeal suturing performance and safety while decreasing workload in the operating room. Robotic suturing showed a better learning curve comparison score than laparoscopic suturing (P < 0.001). Besides, participants' workload was significantly lower with robotic suturing than with laparoscopic suturing (P < 0.001). In conclusion, robotic assistance could lead to improved novice suturing performance, limit the number of inadvertent injuries to structures outside the operating field and decrease operator workload in a live animal model.
Concerns have been raised with regard to the high rate of anastomotic leakage after a laparoscopic low anterior resection for the treatment of rectal cancer. Many relevant clinical factors related to anastomotic leakage have been reported [30-35]. One of the most important issues regarding anastomotic leakage in laparoscopic surgery is known as the stapled rectal resection [31, 32]. Perpendicular application of endostaples to the proposed resection line of the rectum is very difficult; therefore, the number of endostaples may be increased for complete transection of the rectum, which might be related to anastomotic leakage when anastomosis is performed using the double stapled method. Kim et al. [32] reported that the number of linear endostapler firings was significantly related to anastomotic leakage in men. The robotic system might help to reduce the number of endostapler firings, or anastomosis with one stapler may be more easily performed [17]. Following retraction of the specimen through the anal canal, a purse-string suture can be applied to the proximal end of the colon, and anastomosis can be safely performed by using a single circular stapler, which can subsequently be expected to reduce the rate of anastomosis leakage. In addition, a reinforcement suture can be easily applied to the corner of the anastomosis after a double stapling technique using the Endowrist function, which would be very difficult when using a laparoscopic camera system and laparoscpic instruments. Based on these concepts, we believe that the robotic system can reduce the problem of anastomotic leakage in a low anterior resection.
TME has been emphasized by Dr. Heald and advocated by many colorectal surgeons. Originally, he emphasized that the rectum and the mesorectum should be removed as an intact unit with meticulous sharp pelvic dissection under direct vision, staying between the visceral and the parietal pelvic fascia down to the level of the levator muscle [1, 2]. Sharp dissection under direct vision with good illumination is essential during the period of open TME. In addition, gentle opening of the plane by continuous traction and counter-traction is essential. With robotic pelvic dissection, an excellent stereoscopic view can be obtained with high illumination. In addition, with the robotic system, traction and counter-traction can be easily realized in a narrow pelvic space by using Endowrist. One of the advantages is that the 3rd robotic arm can be used. Steady counter-traction is utilized for exposure of the operative field, and a sharp pelvic dissection may be performed. More precise dissection on the "holy plane" between the presacral fascia and the fascia propria of the rectum can be expected with the robotic system, resulting in higher quality of the TME specimen. In particular, during anterior dissection of the seminal vesicle or vagina from Denonvilliers' fascia, which is regarded as an important part of dissection, the robotic system will be helpful in finding and keeping the correct anatomical plane due to the stability of the camera platform and consistent, steady counter-traction by the 3rd robotic arm. As far as autonomic nerve preservation is concerned, the more magnified view, compared to laparoscopy or the naked eye, provides the ability to identify nerve structures.
Personally, dissection of the lower part of the rectum using robotic techniques will be helpful in gaining access to the operative field in any direction when using the self-controlled camera system, allowing clearer visibility for determining the relationship of the lower rectum to the levator ani muscle. Neurovascular bundles are also clearly visible from the pelvic plexus and are easily separated from the rectum. Sometimes, we can locate the middle rectal artery and then dissect the middle rectal artery and divide it with clipping.
A couple of important issues regarding the anatomy of the rectum and the surrounding structures have been under debate. The presence of the lateral ligament has been actively discussed as a topic of pelvic dissection due to the fact that definition and proper division of the lateral ligaments is an essential step in achieving full mobilization of the rectum. In addition, gentle handling of lateral ligaments has been associated with preservation of sexual and urinary function. During the open TME era, posterior and anterior rectal mobilization was always performed according to anatomical landmarks. The main limiting factor for preventing delivery of the rectum from the pelvic inlet has been the lateral part of the rectum. The presence of lateral ligaments has been debated in previous anatomical studies [36-39]. Pak-art et al. [37] beautifully demonstrated an actual lateral ligament by using a cadaveric hemipelvis dissection and concluded that the lateral ligament was present and that it consisted of loose connective tissue with clusters of small nerves. However, according to other observers, the actual position of the lateral ligament was different. Kinugasa et al. [39] insisted that it was located between the mesorectum and the pelvic wall at the ventrolateral side of the pelvic splanchnic nerves; however, Pak-art et al. [37] observed that it was located at the medial side of the pelvic splanchnic nerves originating from the anterior foramina of S3 and S4. Based on our perspectives, definite ligamentous structures between the rectal wall and the pelvic wall are present, and these structures are usually located at an adjacent structure, such as the pelvic plexus or the middle rectal artery. Therefore, traction and division of the lateral part of the rectum has been emphasized for precise dissection to avoid injury to the pelvic plexus and the middle rectal artery. In the robotic era, with a stereoscopic view and self-controlled camera platform, these areas appear to be more clearly visible. The ligament appears to be only an adhesion between the pelvic plexus and the mesorectal fascia of the rectum. Careful and precise dissection should be performed in this area, and the rectum should be mobilized carefully and separated from the pelvic plexus. If the middle rectal artery is detected during surgery, careful ligation or cauterization under robotic assistance is not difficult. The question of whether or not the lateral ligament is present is no longer discussed because the interesting points of the past are no longer a secret.
The sympathetic paraaortic nerve plexux and the superior hypogastric nerve plexus have usually been found around the origin of the inferior mesenteric artery. Injury to these nerves results in sexual dysfunction, such as retrograde ejaculation [40]. Under robotic view, we were able to obtain some benefits with regard to differentiation of nerves and lymphatics from other structures because these nerve structures may be more clearly visible. Practical important points for preservation of the pelvic plexus and neurovascular bundles lie in two steps. The first step is careful separation of the lateral rectal wall with proper traction from the pelvic plexus (coarse and flat meshwork), which consists of the parasympathetic nerve from the sacral foramen. The second step is a precise incision on the Denonvillers' fascia at the seminal vesicle. Dissection behind the plane of the Denonvilliers' fascia is more helpful for preservation of the neurovascular bundles from the genital organs because these neurovascular bundles usually run along the lateral corner of the seminal vesicle and Denonvilliers' fascia will continue to the pelvic plexus. Dr. Kinugasa's studies using cadavers have shown the important relationship between Denonvilliers' fascia and neurovascular bundles [39].
Denonvilliers' fascia was defined as a thick connective tissue layer extending along the long course behind the prostate capsule, in addition to the seminal vesicle. This fascia ended at the plexus area, and one of the lateral continuations of Denonvilliers' fascia extended dorsolaterally and separated the mesorectum from the urogenital neurovascular bundle. It primarily invaded the superior part of the pelvic plexus at the lateral edge of the TME plane. Therefore, the dissection plane should be kept along the plane behind the Denonvilliers' fascia. In addition, Wallner et al. [41], based on cadaveric dissection and clinical case analysis, insisted that fecal and urinary incontinence after a TME was possible due to levator ani nerve disruption. They observed that the levator ani nerve could be disrupted during pelvic dissection of the lower part at the pelvic floor, especially during separation of the mesorectal fascia from the parietal fascia. Therefore, an appropriate surgical plane should be more medial to the parietal pelvic fascia covering the levator ani nerve and the levator ani muscle between the posterior wall of the rectum and the sacrum. Those delicate neurovascular structures are more visible when using a robotic 3-dimensional stereoscopic viewing system.
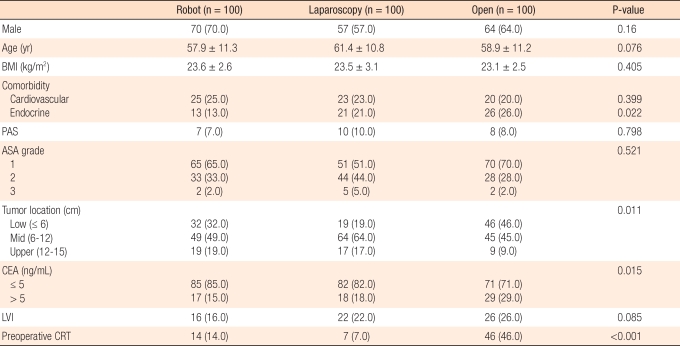
We have investigated short term clinical outcomes and pathologic results between open, laparoscopic, and robotic surgery for the treatment of rectal cancer. About 100 patients in each group were enrolled from January 2008 to December 2009. The proportion of patients with distal rectal cancer and the number of patients who received preoperative chemoradiation therapy was higher in the open surgery group than in the laparoscopy or robotic group. Age and sex distribution did not differ between the groups, and BMI and other factors were same (Table 1).
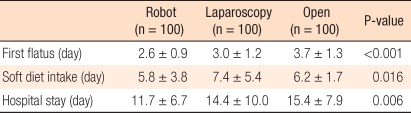
The conversion rate was 2% in the robotic group and 3% in the laparoscopy group. The mean operative time was longer in the robotic group than in the open and laparoscopy groups. The circumferential resection margin's positive rate and the mean of distal resection margin did not differ between the groups. However, the total number of retrieved lymph nodes was larger in the open group than in the laparoscopy or the robotic group (Table 2). The robotic group showed better short-term clinical outcomes than the laparoscopy or the open group in respect to the first flatus, soft diet intake, and hospital stay (Table 3).
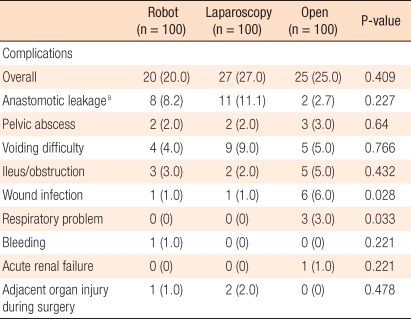
Surgical site infection and pulmonary complications were more common in the open group than in the laparoscopy or the robotic group. The rate of anastomotic leakage was 8.2% in the robotic group, 11.1% in the laparoscopy group, and 2.7% in the open group, but these differences were not statistically significant (P = 0.227) (Table 4).
Our hypothesis is that meticulous, precise dissection with steady counter traction by a robotic arm could make visualizing and preserving the pelvic autonomic nerves better. Patients enrolled in the study included 39 patients in the laparoscopy group and 30 patients in the robotic group. We investigated patients using a questionnaire to assess voiding and sexual function before and after TME at 1, 3, 6, and 12 months after surgery. The IPSS score had decreased to normal at 3 months after TME in the robotic group, and voiding volume appeared to be normal at 1 month after TME in the robotic group. Erectile function was measured as IIEF at 1, 3, 6, and 12 months after TME, and showed a return to normal level at 6 month in the robotic surgery group. Based on our experiences, overall voiding and voiding function will be recovered and come back safely and more rapidly with robotic surgery than with laparoscopy.
The main limitation of propagation of robotic TME in the treatment of rectal cancer in Korea is the high cost. We analyzed and compared total payments and total burdens by patients between robotic, laparoscopy, and open surgery for the treatment of rectal cancer. We analyzed a total of 30 patients (10 patients in each group) who underwent surgery at our hospital. They recovered and were discharged on time without any complications.
The means of total hospital costs were 14,080 USD in robotic surgery, 9,120 USD in laparoscopy surgery and 8,386 USD in open surgery (P < 0.01). Total cost burdens by patients were 11,886 USD in robotic surgery, 3,989 USD in laparoscopy surgery and 3,472 USD in open surgery (P < 0.01) (Fig. 3). In Korea, the cost of robotic surgery is not reimbursed by the national insurance system. Therefore, the cost of using robotic systems and related supplies must be paid by the patients themselves.
Of particular interest, Rawlings et al. [42] analyzed costs between the laparoscopy and the robotic groups. In right hemicolectomies, total hospital costs showed no statistically significant difference between laparoscopy and robotic surgery (P = 0.43), even though costs for operating-room time and operating- room supplies were higher in the robotic group than in the laparoscopy group. With respect to sigmoid colectomies, total hospital costs showed no differences between the laparoscopy group and the robotic group (P = 0.735). Although there was no difference in operating-room time cost between the two groups, the operating-room supply costs were higher in the robotic group than in the laparoscopy group.
The robotic system currently in use still has technical limitations [43]. First, with respect to visual systems, 3-dimensional vision is only appreciated by the operating surgeon, not by the surgical assistant. Though surgeons can magnify the target organ by using the camera system, it is difficult to zoom-out for an overview during surgery, which is sometimes essential for multi-quadrant colorectal surgery. A second limitation concerns the robotic system. Collisions between robotic arms are inevitable owing to the huge size of robotic arms compared to the small abdominal belly, especially for low body-mass-index patients. Loss of haptic sensation at the instrument tip is also problematic. Robotic surgery cannot even offer the indirect or direct touch sensation that laparoscopic or open surgery does. Third, relatively few robotic instruments are available to colorectal surgeon. There are no suction, irrigation, or stapling devices for robotic surgery. In the current system, these functions can only be performed by the assisting doctor. However, progress in mechanical fields is continuing. Therefore, we expect these technical limitations of the robotic system to be overcome with further advances in technology in the near future.
Anyone who has had the opportunity to work with a robotic surgical system can appreciate its advantages in terms of visualization, precision, and ergonomics, compared with the conventional laparoscopic system or open surgery. However, many socioeconomic issues remain to be solved in the future. In addition, we need more concrete evidence regarding the merits for both patients and surgeons, as well as the merits over conventional laparoscopic techniques. Therefore, we need large-scale prospective randomized clinical trials to prove the potential benefits of robot TME for the treatment of rectal cancer. Fortunately, robotic versus laparoscopic resection for rectal cancer (ROLARR) clinical trials have now begun.
The most important point is that a complete understanding of fundamental surgical techniques for the treatment of rectal cancer should be kept in mind because the extent of surgery and the quality of surgery must be the same regardless of the method of surgery, either open, laparoscopy, or robotic. In addition, a multimodality team approach should be kept in mind for improving oncologic outcomes and quality of life.
With technical advancement, evolutionary change of surgical treatment modality should be achieved gradually. We must carefully evaluate cost-effectiveness and validate the safety of new modalities. With continued technological improvements, robotic surgery should become cheaper, more efficient, and more automated. In the near future, robotic surgery may prove advantageous and may revolutionize minimally invasive surgery and radically change the paradigm of laparoscopy. Using these achievements of technical progress, we expect lowering the conversion rate, autonomic nerve preservation and completeness of TME to be more easily achieved by using a robotic system for the treatment of rectal cancer.
References
1. Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery: the clue to pelvic recurrence? Br J Surg 1982;69:613–616. PMID: 6751457.


2. MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet 1993;341:457–460. PMID: 8094488.


3. Maslekar S, Sharma A, Macdonald A, Gunn J, Monson JR, Hartley JE. Mesorectal grades predict recurrences after curative resection for rectal cancer. Dis Colon Rectum 2007;50:168–175. PMID: 17160574.


4. Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet 1986;2:996–999. PMID: 2430152.


5. Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc 1991;1:144–150. PMID: 1688289.

6. Berends FJ, Kazemier G, Bonjer HJ, Lange JF. Subcutaneous metastases after laparoscopic colectomy. Lancet 1994;344:58PMID: 7912321.

7. Lacy AM, Garcia-Valdecasas JC, Delgado S, Castells A, Taura P, Pique JM, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet 2002;359:2224–2229. PMID: 12103285.


8. Clinical outcomes of surgical therapy study group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004;350:2050–2059. PMID: 15141043.


9. Colon Cancer Laparoscopic or Open Resection Study Group. Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol 2009;10:44–52. PMID: 19071061.


10. Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol 2007;25:3061–3068. PMID: 17634484.


11. Maeso S, Reza M, Mayol JA, Blasco JA, Guerra M, Andradas E, et al. Efficacy of the Da Vinci surgical system in abdominal surgery compared with that of laparoscopy: a systematic review and meta-analysis. Ann Surg 2010;252:254–262. PMID: 20622659.


12. Pigazzi A, Garcia-Aguilar J. Robotic colorectal surgery: for whom and for what? Dis Colon Rectum 2010;53:969–970. PMID: 20551745.


13. Schwab KE, Dowson HM, Van Dellen J, Marks CG, Rockall TA. The uptake of laparoscopic colorectal surgery in Great Britain and Ireland: a questionnaire survey of consultant members of the ACPGBI. Colorectal Dis 2009;11:318–322. PMID: 18573117.


14. Rea JD, Cone MM, Diggs BS, Deveney KE, Lu KC, Herzig DO, et al. Utilization of laparoscopic colectomy in the United States before and after the clinical outcomes of surgical therapy (COST) study group trial In: The American Society of Colon and Rectal Surgeons 2010 Annual Meeting; 2010 May 15-9; Minneapolis, MN, USA.
15. Bege T, Lelong B, Esterni B, Turrini O, Guiramand J, Francon D, et al. The learning curve for the laparoscopic approach to conservative mesorectal excision for rectal cancer: lessons drawn from a single institution's experience. Ann Surg 2010;251:249–253. PMID: 20040854.


16. Park IJ, Choi GS, Lim KH, Kang BM, Jun SH. Multidimensional analysis of the learning curve for laparoscopic colorectal surgery: lessons from 1,000 cases of laparoscopic colorectal surgery. Surg Endosc 2009;23:839–846. PMID: 19116741.


17. Choi GS, Park IJ, Kang BM, Lim KH, Jun SH. A novel approach of robotic-assisted anterior resection with transanal or transvaginal retrieval of the specimen for colorectal cancer. Surg Endosc 2009;5 14 [Epub]. DOI: 10.1007/s00464-009-0484-5.

18. Park JS, Choi GS, Lim KH, Jang YS, Jun SH. S052: a comparison of robot-assisted, laparoscopic, and open surgery in the treatment of rectal cancer. Surg Endosc 2010;6 15 [Epub]. DOI: 10.1007/s00464-010-1166-z.

19. Pigazzi A, Ellenhorn JD, Ballantyne GH, Paz IB. Robotic-assisted laparoscopic low anterior resection with total mesorectal excision for rectal cancer. Surg Endosc 2006;20:1521–1525. PMID: 16897284.


20. Baik SH, Ko YT, Kang CM, Lee WJ, Kim NK, Sohn SK, et al. Robotic tumor-specific mesorectal excision of rectal cancer: short-term outcome of a pilot randomized trial. Surg Endosc 2008;22:1601–1608. PMID: 18270772.


21. Choi DJ, Kim SH, Lee PJ, Kim J, Woo SU. Single-stage totally robotic dissection for rectal cancer surgery: technique and short-term outcome in 50 consecutive patients. Dis Colon Rectum 2009;52:1824–1830. PMID: 19966627.


22. Park YA, Kim JM, Kim SA, Min BS, Kim NK, Sohn SK, et al. Totally robotic surgery for rectal cancer: from splenic flexure to pelvic floor in one setup. Surg Endosc 2010;24:715–720. PMID: 19688388.


23. Baek JH, Pastor C, Pigazzi A. Robotic and laparoscopic total mesorectal excision for rectal cancer: a case-matched study. Surg Endosc 2010;7 07 [Epub]. DOI: 10.1007/s00464-010-1204-x.

24. Park JS, Choi GS, Lim KH, Jang YS, Jun SH. Robotic-assisted versus laparoscopic surgery for low rectal cancer: case-matched analysis of short-term outcomes. Ann Surg Oncol 2010;17:3195–3202. PMID: 20589436.


25. Baik SH, Kwon HY, Kim JS, Hur H, Sohn SK, Cho CH, et al. Robotic versus laparoscopic low anterior resection of rectal cancer: short-term outcome of a prospective comparative study. Ann Surg Oncol 2009;16:1480–1487. PMID: 19290486.


26. Bianchi PP, Ceriani C, Locatelli A, Spinoglio G, Zampino MG, Sonzogni A, et al. Robotic versus laparoscopic total mesorectal excision for rectal cancer: a comparative analysis of oncological safety and short-term outcomes. Surg Endosc 2010;24:2888–2894. PMID: 20526623.


27. Baek JH, McKenzie S, Garcia-Aguilar J, Pigazzi A. Oncologic outcomes of robotic-assisted total mesorectal excision for the treatment of rectal cancer. Ann Surg 2010;251:882–886. PMID: 20395863.


28. Van Koughnett JA, Jayaraman S, Eagleson R, Quan D, Van Wynsberghe A, Schlachta CM. Are there advantages to robotic-assisted surgery over laparoscopy from the surgeon's perspective? J Robot Surg 2009;3:79–82.


29. Stefanidis D, Wang F, Korndorffer JR Jr, Dunne JB, Scott DJ. Robotic assistance improves intracorporeal suturing performance and safety in the operating room while decreasing operator workload. Surg Endosc 2010;24:377–382. PMID: 19536599.


30. Akiyoshi T, Kuroyanagi H, Oya M, Konishi T, Fukuda M, Fujimoto Y, et al. Factors affecting the difficulty of laparoscopic total mesorectal excision with double stapling technique anastomosis for low rectal cancer. Surgery 2009;146:483–489. PMID: 19715805.


31. Ito M, Sugito M, Kobayashi A, Nishizawa Y, Tsunoda Y, Saito N. Relationship between multiple numbers of stapler firings during rectal division and anastomotic leakage after laparoscopic rectal resection. Int J Colorectal Dis 2008;23:703–707. PMID: 18379795.


32. Kim JS, Cho SY, Min BS, Kim NK. Risk factors for anastomotic leakage after laparoscopic intracorporeal colorectal anastomosis with a double stapling technique. J Am Coll Surg 2009;209:694–701. PMID: 19959036.


33. Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery 2010;147:339–351. PMID: 20004450.


34. Huh JW, Kim HR, Kim YJ. Anastomotic leakage after laparoscopic resection of rectal cancer: the impact of fibrin glue. Am J Surg 2010;199:435–441. PMID: 19481197.


35. Bertelsen CA, Andreasen AH, Jorgensen T, Harling H. Danish Colorectal Cancer Group. Anastomotic leakage after anterior resection for rectal cancer: risk factors. Colorectal Dis 2010;12:37–43. PMID: 19175624.

36. Lin M, Chen W, Huang L, Ni J, Yin L. The anatomy of lateral ligament of the rectum and its role in total mesorectal excision. World J Surg 2010;34:594–598. PMID: 20052469.


37. Pak-art R, Tansatit T, Mingmalairaks C, Pattana-arun J, Tansatit M, Vajrabukka T. The location and contents of the lateral ligaments of the rectum: a study in human soft cadavers. Dis Colon Rectum 2005;48:1941–1944. PMID: 16175322.


38. Jones OM, Smeulders N, Wiseman O, Miller R. Lateral ligaments of the rectum: an anatomical study. Br J Surg 1999;86:487–489. PMID: 10215819.


39. Kinugasa Y, Murakami G, Uchimoto K, Takenaka A, Yajima T, Sugihara K. Operating behind Denonvilliers' fascia for reliable preservation of urogenital autonomic nerves in total mesorectal excision: a histologic study using cadaveric specimens, including a surgical experiment using fresh cadaveric models. Dis Colon Rectum 2006;49:1024–1032. PMID: 16732487.


40. Kim NK, Aahn TW, Park JK, Lee KY, Lee WH, Sohn SK, et al. Assessment of sexual and voiding function after total mesorectal excision with pelvic autonomic nerve preservation in males with rectal cancer. Dis Colon Rectum 2002;45:1178–1185. PMID: 12352233.


41. Wallner C, Lange MM, Bonsing BA, Maas CP, Wallace CN, Dabhoiwala NF, et al. Causes of fecal and urinary incontinence after total mesorectal excision for rectal cancer based on cadaveric surgery: a study from the Cooperative Clinical Investigators of the Dutch total mesorectal excision trial. J Clin Oncol 2008;26:4466–4472. PMID: 18802159.


42. Rawlings AL, Woodland JH, Vegunta RK, Crawford DL. Robotic versus laparoscopic colectomy. Surg Endosc 2007;21:1701–1708. PMID: 17353988.


Fig. 1
Penetration rate of laparoscopic surgery for total colorectal cancer in Korea (Courtesy of Prof. K. Y. Lee, M.D., Kyung Hee University School of Medicine, Seoul, Korea).
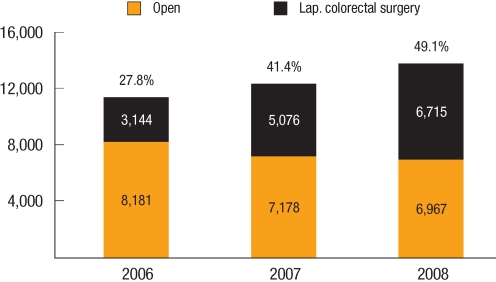
Fig. 2
Penetration rate of laparoscopic surgery for rectal cancer in Korea (Courtesy of Prof. K. Y. Lee, M.D., Kyung Hee University School of Medicine, Seoul, Korea).
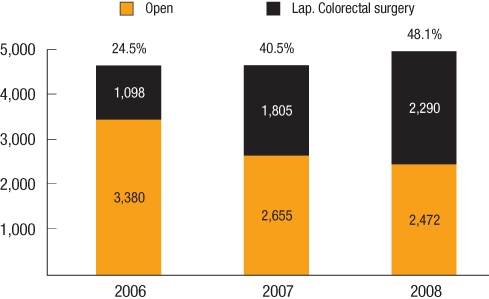
Fig. 3
Comparison of total cost under Korea medical insurance system: robot vs. laparoscopy vs. open surgery for rectal cancer.