Transanal Endoscopic Microsurgery for Patients With Rectal Tumors: A Single Institution's Experience
Article information
Abstract
Purpose
The purpose of this study was to look at our complication rates and recurrence rates, as well as the need for further radical surgery, in treating patients with benign and early malignant rectal tumors by using transanal endoscopic microsurgery (TEM).
Methods
Our study included 130 patients who had undergone TEM for rectal adenomas and early rectal cancer from December 2009 to December 2015 at the Department of Surgical Oncology, National Cancer Institute, Lithuania. Patients underwent digital and endoscopic evaluation with multiple biopsies. For preoperative staging, pelvic magnetic resonance imaging or endorectal ultrasound was performed. We recorded the demographics, operative details, final pathologies, postoperative lengths of hospital stay, postoperative complications, and recurrences.
Results
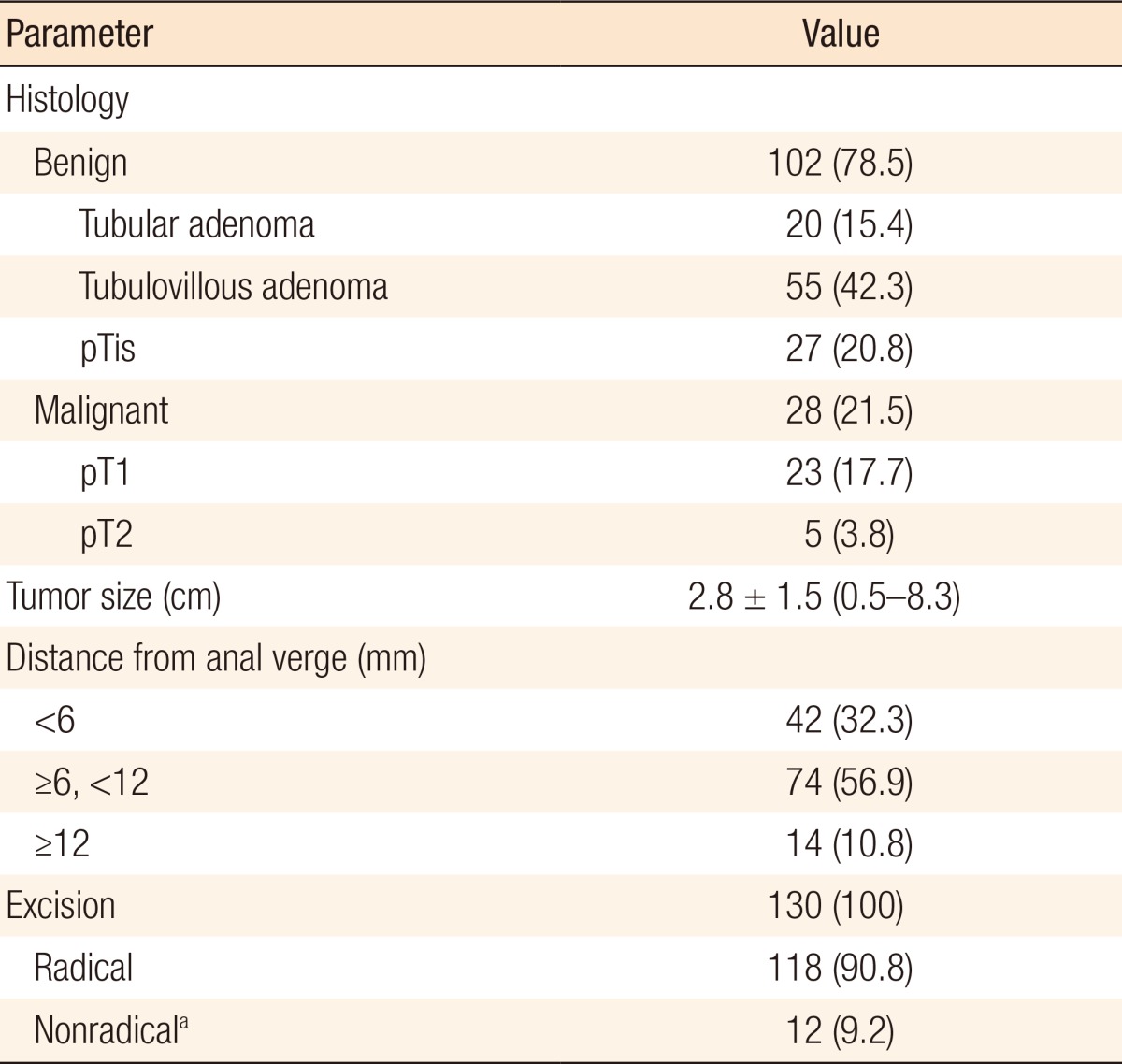
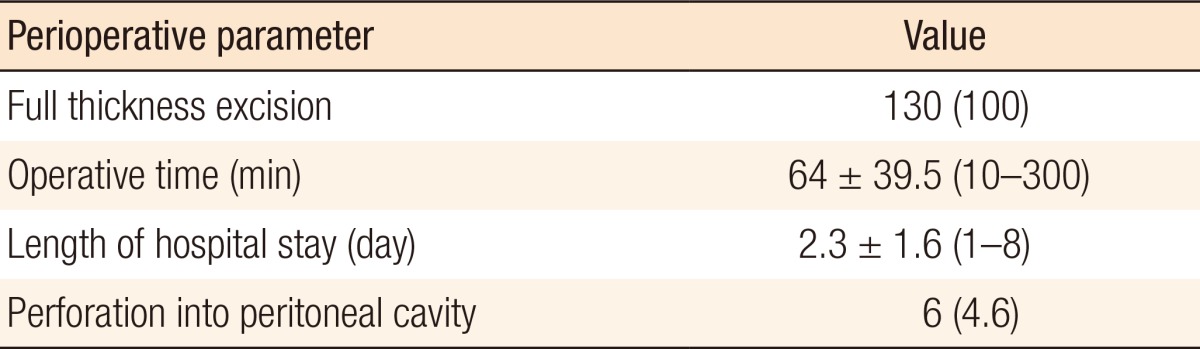
The average tumor size was 2.8 ± 1.5 cm (range, 0.5–8.3 cm). 102 benign (78.5%) and 28 malignant tumors (21.5%) were removed. Of the latter, 23 (82.1%) were pT1 cancers and 5 (17.9%) pT2 cancers. Of the 5 patients with pT2 cancer, 2 underwent adjuvant chemoradiotherapy, 1 underwent an abdominoperineal resection, 1 refused further treatment and 1 was lost to follow up. No intraoperative complications occurred. In 7 patients (5.4%), postoperative complications were observed: urinary retention (4 patients, 3.1%), postoperative hemorrhage (2 patients, 1.5%), and wound dehiscence (1 patient, 0.8%). All complications were treated conservatively. The mean postoperative hospital stay was 2.3 days.
Conclusion
TEM in our experience demonstrated low complication and recurrence rates. This technique is recommended for treating patients with a rectal adenoma and early rectal cancer and has good prognosis.
INTRODUCTION
Globally, colon and rectal cancer (CRC) rank third for the incidence of cancer and fourth for cancer deaths in 2013. For developed countries, it is ranked second for incidence and mortality, and in developing countries, it is ranked fourth for incidence and mortality [1]. The mortality is showing a tendency to decrease [2]. In addition, colorectal adenomas are known to lead to colorectal cancer especially when they possess a villous component and grow larger [34].
Transanal endoscopic microsurgery (TEM) is a minimally invasive procedure requiring a complex setup that provides surgical access to the rectum. Oncologic results are adequate, and morbidity and mortality are low when the technique is applied appropriately. TEM allows magnified stereoscopic visualization, as well as manipulability, in a narrow space. Alternatively, today, total mesorectal excision (TME) is a widely accepted routine technique in rectal, including early stage, cancer surgery [5]. However, radical surgery is sometimes followed by perioperative mortality (2%–3%) and morbidity (20%–30%): anastomotic leakage, sepsis, per-manent or temporary stoma, and urinary, sexual and/or bowel dysfunction [6]. In contrast, the morbidity of TEM is reported in the literature to be 4% to 29% [7]. The most common complications after TEM are bleeding, dehiscence, peritoneal entry, conversion to a laparotomy, urinary retention, and fecal soiling. TEM is recommended for all patients with benign lesions and early rectal cancer with good oncological criteria (well to moderately differentiated, confined to the submucosa: T1, no lymph-node invasion, no lymphovascular or perineural invasion, no mucinous or signet cell component) from 4 to 15 cm from the dentate line that occupy no more than 30% of the bowel circumference, are no larger than 3 cm in size, and are mobile [8]. The purpose of this study was to look at our complication rates and recurrence rates, as well as the need for further radical surgery, when using TEM to treat patients with benign and early malignant rectal tumors.
METHODS
The study design included 130 patients who had undergone TEM for rectal adenomas, carcinoids and early rectal cancer from December 2009 to December 2015 at the Department of Surgical Oncology, National Cancer Institute, Lithuania. The adenomas were declared to be inappropriate for snare excision. All patients were evaluated preoperatively according to a standard protocol, including physical and endoscopic examination and tumor biopsy, and some patients underwent endorectal ultrasonography or magnetic nuclear resonance imaging (MRI). Tumor location was defined as the distance from the anal verge to the lower edge of the tumor, and the diameter was defined as the largest diameter of the tumor. We recorded the demographics, operative details, final pathologies, postoperative lengths of hospital stay, postoperative complications, and recurrences.
All patients underwent bowel preparation with a polyethylene-glycol solution and were given preoperative intravenous antibiotics. All TEM procedures were performed under general anesthesia in either the lithotomy, prone jack-knife, or lateral decubitus position, depending on the exact location of the tumor. Standard G. Buess TEM equipment available from Richard Wolf Medical Instruments (Vernon Hills, IL, USA) was used. Full thickness ex-cision with a 1-cm safety margin (for early rectal cancer or a potentially malignant tumor) and a 5-mm safety margin for benign lesions was attempted, followed by closure of the rectal wall defect in one-layer by using silver clips to run a Monocryl 3-0 suture.
Follow-up was performed under our institutional guidelines: for benign lesions, proctoscopy after 3 months and colonoscopy 1 year later, 3 years later for a tubulovillous adenoma; for a tubular adenoma, proctoscopy after 3 months and colonoscopy 1 year and 3 years after surgery; for malignant lesions, an abdominal computed tomography (CT) or ultrasound scan, a pelvic MRI, and a chest X-ray every 3 months for 2 years, then once a year thereafter. Data were entered, calculated and analyzed in Microsoft Office Excel 2013 (Microsoft Corp., Redmond, WA, USA). We report most analyses as simple descriptive statistics with a standard deviation unless otherwise specified.
RESULTS
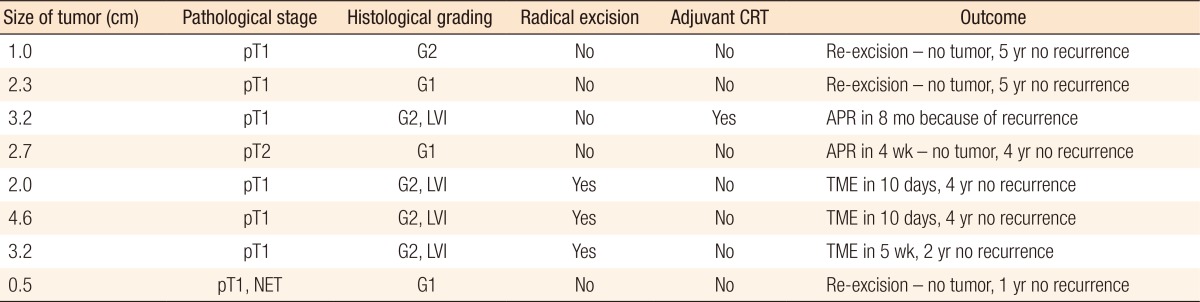
Of the 130 eligible patients, 63 (48%) were male and 67 (52%) were female patients. The mean age of our patients was 67.7 ± 10.9 years (range, 31–93 years). The average tumor size was 2.8 ± 1.5 cm (range, 0.5–8.3 cm). The rectum was subdivided into 3 parts: distal (0–6 cm from the anal verge), middle (6–12 cm), and proximal (>12 cm). Most tumors (74 [56.9%]) were located in the midrectum, 14 (10.8%) in the proximal rectum, and 42 (32.3%) in the distal rectum (Table 1). 102 benign (78.5%) and 28 malignant tumors (21.5%) were removed. Of the latter 28, 23 (82.1%) were pT1 cancers and 5 (17.9%) pT2 cancers. Two patients with pT2 cancer underwent adjuvant chemoradiotherapy, one underwent an abdominoperineal resection, one refused further treatment, and one was lost to follow up (Table 2). Six patients in the cancer group did not undergo radical procedures (margin was less than 1 cm), 3 underwent a re-excision, but the pathology report did not show a residual tumor, 2 underwent radical surgery, and 1 patient chose a watch and wait policy (he had no recurrence after 1 year).
No intraoperative complications were encountered. In 7 of the 130 cases (5.4%), postoperative complications were observed: urinary retention (4 of 130 cases, 3.1%), postoperative hemorrhage (2 of 130 cases, 1.5%), and wound dehiscence (1 of 130 cases, 0.8%). All complications were treated conservatively. The mean postoperative hospital stay was 2.3 days, and the mean operative time was 64 ± 39.5 minutes (range, 10–300 minutes). We had 6 cases of peritoneal entry, none of which needed conversion (Table 3). Two of the 130 cases (1.5%) were found to have a recurrence (1 in the adenoma group and 1 in the pT2 cancer group) during the follow-up period. The mean follow-up time was 32.8 ± 19.1 months (range, 7–67.5 months).
DISCUSSION
Nowadays, TEM has become a very viable alternative to the transanal approach in the treatment of patients with rectal adenomas or early rectal cancer. Various articles demonstrating different postoperative morbidity rates ranging from 11.8% to 17% for local excision (LE) [910], 7.7%, to 21% for TEM [1112], and 18% to 55% for a radical resection [913] have been published. The study of de Graaf et al. [14] was the first to find statistically significant differences when comparing the safeties of LE and TEM; they reported postoperative mortality rates of 5.3% and 10% after TEM and LE, respectively. In our study, we had 7 cases (5.3%) of postoperative morbidity, which is less than the results reported by other authors [1112]. All of those complications were relatively common and easily treatable by using conservative methods; the urinary function for the patients who experienced retention was restored using urinary catheterization, and postoperative bleeding was treated by using intravenous fluid infusions and blood transfusions. Furthermore, not a single TEM procedure was converted to a laparoscopic or open surgery, thus showing its suitability for treating patients with rectal tumors.
TEM has also been found to have lower recurrence rates, with recurrence rates for LE ranging from 4% to 57% and those for TEM ranging from 3% to 16% [14]. Among the main factors that affect recurrence after these operations are incomplete excision and fragmentation of acquired specimens [1516]. TEM was shown to be more likely to provide clear resection margins and less fragmentation than the transanal approach, which could be the reason for the lower recurrence rates with TEM. Our results regarding the fragmentation rate or positive resection margin were similar to those described by other authors: we found 9.2% fragmentation or positive or unclear resection margins, compared to the 1.4% fragmentation and the 12% unclear resection margins reported by de Graaf et al. [14] and the 6% fragmentation and the 10% unclear resection margins reported by Moore et al. [10].
Concerning the rate of positive or unclear resection margins, how many of the specimens in the “unclear” category were actually positive or negative is unclear. The fact that the main surgical method for excising tumors was coagulation, which can distort margins and make them unclear, must be taken into account; therefore, the actual number of positive resection margins is most likely lower than reported. In either case, the recurrence rate was not affected by these findings; we found two (2 of 130, 1.5%) local recurrences (1 in the benign tumor group and 1 in the malignant tumor group) during our follow-up, which was lower than that described by other authors and proves the effectiveness of this method for rectal tumor removal [151718]. Another concern is systemic recurrence for early rectal cancer. In the literature, recurrence rates from 0% [19] to 20.5% [20] can be found. In the latter study, the authors could not give reasons for the high numbers of local recurrences. The worse results for recurrence may be explained by poor prognostic features: lymphatic invasion, tumor budding (sprouting), vascular involvement, a poorly differentiated tumor, and the depth of tumor invasion. That even superficial T1 cancer carries a risk of lymph-node metastasis is well documented. Son et al. [21] found that the rate of lymph-node metastasis in patients with sm1 cancer was as large as 3.1%. Nakadoi et al. [22] showed that tumor invasion of less than 1,000 µm still carried a lymph-node metastasis risk of 2.2%. In T2 cancer, the risk of lymph-node metastasis is even higher – up to 25.7% [23]. A few recent systemic reviews and meta-analyses showed that for patients with T1 cancer, the distant metastasis rate and the overall survival and the disease-free survival rates did not differ between the TEM and the radical surgery groups, although the local recurrence rate after TEM was higher than that after radical surgery. The complication rate was significantly higher in the radical surgery group [2425].
Entry into the peritoneal cavity during TEM was not associated with an increased risk of complications. We and other authors do not consider it to be a cause of complications [2627]. We had 6 patients (6 of 130, 5.4%) whose peritoneal cavity was entered during surgery. None of them required conversion; we sutured the defect with a single-layer suture.
In conclusion, previous studies at our clinic had already shown TEM to be an alternative to the transanal approach when treating patients with benign and malignant rectal masses, and since those studies, it has become the procedure of choice for the treatment of patients with rectal tumors [2829]. Acceptable short-term outcomes—low recurrence rates and low complication rates after the surgery—allow us to conclude that TEM is an effective and safe method for the treatment of patients with rectal tumors.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.