- Search
Abstract
Purpose
Although rectal cancer is a very common malignancy and has an improved cure rate in response to oncological treatment, research on rectal-cancer survivors' sexual function remains limited. Sexual dysfunction (SD) after rectal cancer treatment was measured, and possible predisposing factors that may have an impact on the development of this disorder were identified.
Methods
Patients undergoing curative rectal cancer surgery from January 2012 to September 2013 were surveyed using questionnaires. The female sexual function index or the International Index of Erectile Function was recorded. A multiple logistic regression was used to test associations of clinical factors with outcomes.
Results
Fifty-six men (56%) and 28 women (44%) who completed the questionnaire were included in the study. A total of 76 patients of the 86 patients (90.5%) with the diagnosis of rectal cancer who were included in this study reported different levels of SD after radical surgery. A total of 64 patients (76%) from the whole cohort reported moderate to severe SD after treatment of rectal cancer. Gender (P = 0.011) was independently associated with SD. Female patients reported significantly higher rates of moderate to severe SD than male patients. Patients were rarely treated for dysfunction.
Conclusion
Sexual problems after surgery for rectal cancer are common, but patients are rarely treated for SD. Female patients reported higher rates of SD than males. These results point out the importance of sexual (dys)function in survivors of rectal cancer. More attention should be drawn to this topic for clinical and research purposes.
The main treatment goals for rectal cancer are oncological cure and overall survival. With improved oncological results, functional results such as fecal continence and urinary and sexual function become increasingly important [1]. Sexual dysfunction (SD) is a common complication of rectal cancer surgery [2]. Many patients experience deterioration in sexual function, consisting of erectile dysfunction (ED) in men and vaginal dryness and dyspareunia in women, after rectal cancer treatment [3]. Reduced sexual function is associated with lower quality of life in cancer survivors [4]. Although rectal cancer is a common malignancy and has an improved oncological cure rate, research on sexual function after rectal cancer surgery remains limited [5].
The aim of this study was to identify the frequency of SD after rectal cancer treatment. Also, possible predisposing factors that may have an impact on the development of SD are discussed.
The study was planned as a cross-sectional, single-arm cohort study. All cases of rectal cancer that involved surgery with curative intent at The Marmara University School of Medicine, Pendik Training & Research Hospital, from 2012 to 2013 were selected from a database of all colorectal cancer operations. The Research Ethics Committee of Marmara University approved the study, and all patients signed a written informed consent form before participation in the study.
Inclusion criteria were patients with rectal cancer who underwent a radical resection for any stage or rectal cancer. Survivors treated with surgery (with or without [neo]adjuvant therapy) were included. Exclusion criteria were an inability to obtain informed consent and transanal excision of the primary tumor.
Sexual function after treatment was measured using the validated questionnaire the female sexual function index for females and the International Index of Erectile Function (IIEF) for males. Validated self-reported psychometric questionnaires, such as IIEF and the Index of Female Sexual Function (IFSF), helped us to assess the impact of a specific treatment modality by evaluating different sexual function domains [6, 7]. The original IIEF, which consists of 15 items and 5 domains, is a psychometrically-valid and reliable instrument for ED assessment that was developed through consultations with an international panel of experts [6]. Each IIEF item is scored on a 5-point ordinal scale, where lower values represent poorer sexual function. Thus, a response of 1 for a question was considered the least functional whereas a response of 5 was considered the most functional. However, a need exists for a simpler patient-administered tool for the diagnosis of ED for easy use by physicians in clinical settings. An abbreviated version of the IIEF, designated as the IIEF-5, has been developed and validated as a diagnostic tool for ED [8]. According to the IIEF-5, ED can be classified into five severity levels, ranging from none (22-25), to mild (17-21), mild-to-moderate (12-16), moderate (8-11), and severe (5-7).
The IFSF, a 9-item questionnaire, has been developed as a brief, multidimensional self-reporting instrument for assessing the key dimensions of sexual function in women [7]. Specific domains analyzed in the IFSF included quality of sexual intercourse, desire, overall satisfaction with sexual function, orgasm, lubrication, and clitoral sensation. Specific questions analyzed included the degree of lubrication, the ability to achieve orgasm, and the degree of clitoral sensation, with responses graded on a scale of 1 (almost never or never) to 5 (almost always or always) [7]. A score of 0 indicated no attempt at intercourse. According to the IFSF, SD in women can be classified into four levels, ranging from none (Ōēź35) to mild (26-35), moderate (16-25) and severe (Ōēż15) in the Turkish female population [9]. For the assessment of the possible predisposing factors that may have an impact on the development of SD, we separated the patients into two groups (A and B) according to their SD scores: namely, no and mild SD in one group (group A) and mild-to-moderate, moderate and severe in the other group (group B).
Information on age, gender, histological diagnosis, surgical procedure, pathological TNM stage, (neo)adjuvant treatment, and postoperative complications such as anastomosis leakage, bleeding, complications of ostomy, evisceration, perineal complications, reoperations were obtained from the medical records. These data were obtained for all patients who underwent surgery for rectal cancer between 2012 and 2013 at one center.
Background clinical data were analyzed using the t-test for continuous data and Fisher exact test or the chi-square test for categorical data. Data for the total IIEF and IFSF scores and their domain scores were analyzed using a two-way analysis of variance. A multivariate logistic regression analysis with 95% confidence interval was used to identify factors associated with SD. Data were analyzed using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). All tests were two-sided, and P-values below 0.05 were considered statistically significant.
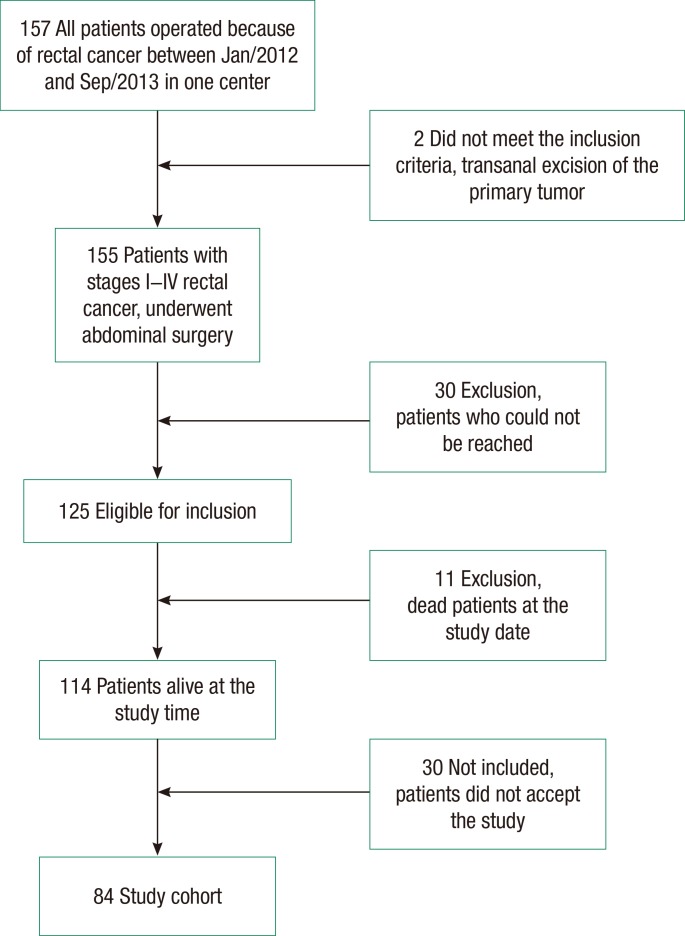
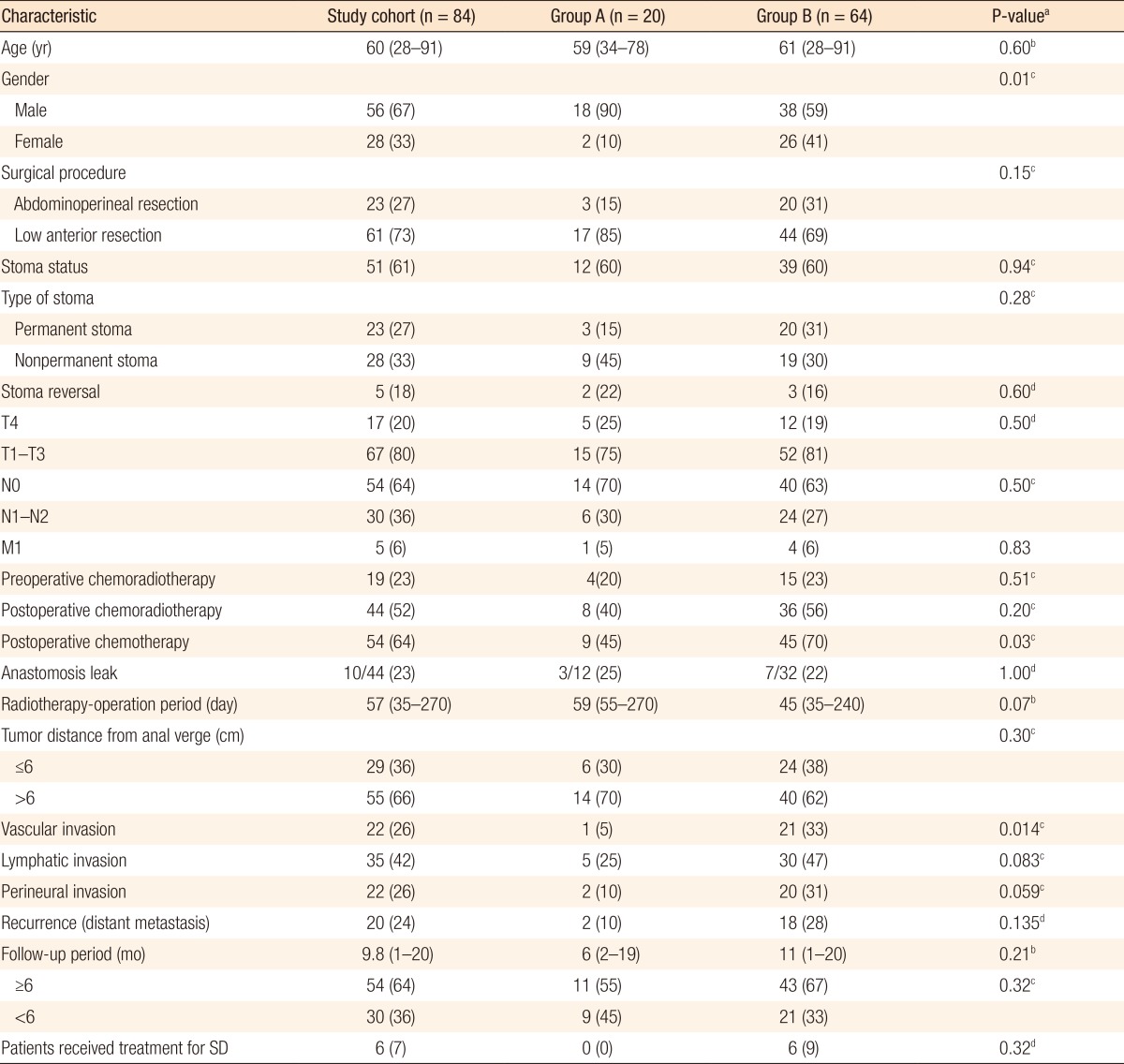
Between January 2012 and September 2013, 157 patients were operated on for rectal cancer in the General Surgery Clinic at Marmara University School of Medicine, Pendik Training & Research Hospital. A transanal excision was done for 2 patients, and a radical resection was performed for 155 patients (abdominoperineal resection for 37 patients, and low anterior resection for 120 patients). Patients who underwent a transanal excision were excluded. We had access to 125 patients who underwent a radical resection with abdominal surgery. Of the 125 patients, 114 were alive, and 11 had died. Thirty patients did not accept participating in the study. Five of those 30 patients did not accept participating in the study because they were not able to come to the hospital. The other 25 patients were not sexually active and, accordingly, did not accept the study. The remaining 84 patients who accepted the study and signed the informed consent forms were invited to the hospital so that evaluate their sexual functions could be evaluation (Fig. 1). All patients' median age was 60 years (28-91 years), and all of them were diagnosed with an adenocarcinoma. The demographic and the clinical characteristics of the patients are shown in Table 1.
In this study, 56 patients (67%) were male. The operative methods were abdominoperineal resection with open surgery in 23 patients (27.4%), low anterior resection without ostomy in 33 (39.3%; open surgery, n = 31; laparoscopic surgery, n = 2), low anterior resection with protective ostomy in 12 (14.3%; open surgery, n = 11; laparoscopic surgery, n = 1), and Hartmann's procedure in 16 patients (19%). The median distance between the anal verge and the tumor was 9 cm (range, 1-15 cm).
More than half of the patients had T3 as a primary tumor stage (51.2%, n = 43). Regional lymph nodes stage was N0 in 51 patients (60.7%). Distant metastasis was observed in 5 patients (6%). Nineteen patients (23%) underwent preoperative chemoradiotherapy. A total of 54 patients (64%) underwent postoperative chemotherapy, including 44 (52%) who received concomitant adjuvant radiotherapy. The median time between neoadjuvant radiotherapy and surgery was 57 days (range, 35-270 days). The median follow-up period for the entire cohort was 9.8 months (range, 1-20 months). Of the 28 patients who had a nonpermanent stoma, 5 patients (18%) underwent a stoma reversal. A total of 54 patients (64%) had a follow-up period of more than 6 months. Anastomotic leakage was diagnosed in 10 patients (23%) who underwent a low anterior resection with anastomosis, but it was necessary to reoperate on and create a diverting ostomy in only 4 patients (9%). The other 6 patients were healed by using antibiotics and observation.
Using the validated IIEF-5 and IFSF questionnaires, 76 patients (90.5%) reported different levels of SD after radical surgery. A total of 64 patients (76%) from the whole cohort reported moderate-to-severe SD after treatment of rectal cancer. Among the male patients, 6 (10.7%) reported no SD, 12 (21.4%) reported mild, 26 (46.4%) reported mild-to-moderate, 7 (12.5%) reported moderate, and 5 (9.0%) reported severe SD. Among the female patients, 1 (3.7%) reported no SD, 1 (3.7%) reported mild, 19 (70.4%) reported moderate, and 6 (22.2%) reported severe SD. Female patients reported significantly higher rates of moderate-to-severe SD than male patients (93% vs. 68%, respectively; P = 0.011). In the univariate analysis, the postoperative adjuvant chemotherapy rate was significantly higher in group B than in group A (70% vs. 45%, respectively; P = 0.039). The vascular invasion rate in the final pathology report was also significantly higher in group A than in group B, (33% vs. 5%, respectively; P = 0.014). In the multivariate logistic regression analysis, the difference in gender between groups A and B was significant (P = 0.018), but the differences in adjuvant chemotherapy and vascular invasion rate were not significant (P = 0.40 and P = 0.051, respectively). Although a high rate of SD was reported by the patients in this study (90.5%), only 6 patients (8%) sought and received treatment for SD.
This study, which included 84 patients with rectal cancer, showed that SD is a common complaint in patients with rectal cancer after radical treatment. The rate of SD was high (90.5%) in this study cohort. Female patients had a significantly higher rate (93%) of SD than male patients (67%) did.
Conventionally, outcome assessments in colorectal cancer include mortality, morbidity, disease recurrence, and long-term survival. However, patient-reported outcomes (e.g., quality of life) are now also regarded as key measurements in assessing outcomes of interventions [10]. Sexuality and intimacy are considered to be important aspects of quality of life [11]. While rectal cancer treatment has clearly produced improved outcomes for the disease, patients are still plagued by the complications and the long-term consequences of their treatment. SD after rectal cancer treatment is common and can have major negative effects on the quality of life. Despite this, it is not often discussed in clinical practice. Patients are unlikely to mention these problems themselves either because they are embarrassed or because they do not relate their symptoms to their rectal cancer treatment [3]. These complications can have a major impact on patients' psychological, social, and emotional functioning, as well as on their overall well-being [12]. This study fills this gap and highlights the less discussed issue of SD [4]; it also supports the need for studies related to SD after rectal cancer treatment.
This study has some limitations that need to be acknowledged. First, this was a cross-sectional study, and no information was known about sexual (dys)function before diagnosis/treatment of cancer, which limited the determination of the effect of cancer diagnosis and treatment on functioning or on the ability to correct for baseline functioning. Prospective studies with an assessment point prior to surgical treatment are warranted. Second, our study has a potential sampling bias. The study population is a "convenience cohort" of surviving rectal cancer patients who were treated in one center. Therefore, results may not be generalizable to all patients undergoing rectal cancer surgery. Procedure subgroups were not randomly assigned and differed in their tumor characteristics as well as their treatments. The final limitation is the small number of patients for evaluating the factors associated with SD after rectal cancer treatment.
SD is a well-recognized complication after total mesorectal excision (TME) and has a negative impact on patients' quality of life. The incidence of SD after TME is reported to be 18%-54% [12, 13, 14], depending on the type of surgery, neoadjuvant treatment especially radiotherapy, and/or quality of dissection [15]. There are several reasons the rate of SD reported in this study appears high (90.5%) compared with other recent studies. First, this study did not exclude patients based on age or preexisting sexual inactivity or dysfunction, exclusions that bias results toward better function. Second, the median follow-up period (9.8 months) may be too short to evaluate patients after treatment for rectal cancer. Nevertheless, rates of long-term ED ranging from 59% to 90% have been reported in other studies [16, 17, 18]. Widely varying rates of SD after rectal surgery have been reported in the literature, and comparisons between studies are difficult due to the different exclusion criteria and outcome measures used in those studies [19, 20].
Unexpectedly, female patients reported significantly higher rates of SD than male patients (93% vs. 67%, respectively; P = 0.011). While a number of studies have examined rates of SD after surgery for rectal cancer, the primary focus has been on the male sexual issue of ED. Research on female rectal cancer survivors' sexual function remains limited, and generally it is ignored during surgery [19, 21, 22]. Moreover, female SD after surgery for rectal cancer has been relatively ignored [13] due in part to the reluctance of women with rectal cancer to respond to questions about their sexuality [16]. Two factors may help explain the relatively higher rates of SD in female than in male patients: (1) nerve preservation is relatively ignored during surgery in females, and (2) early menopause resulting from (neo)adjuvant chemoradiotherapy may lead to SD after treatment. Moreover, women are more likely than men to give up sexual activity after colorectal cancer treatment [16]. Although adjuvant chemotherapy does not increase the risk of male SD [23], adjuvant pelvic radiotherapy or chemoradiation does increase the rate of ED [14]. Rather unexpected was the finding that radiotherapy was not significantly associated with SD. Considering that radiotherapy is one of the most robust findings in the literature, our contradictory finding is noteworthy. The inconsistency with the literature may be explained by the small number of our cohort and by the inclusion of patients with different stages of rectal cancer.
Stoma status and type of resection were not significantly different between the groups with and without SD. Milbury et al. [5] reported that neither having a stoma nor type of resection was significantly related to SD for women. This study showed that most patients did not get treatment for SD. Although one randomized trial showed that sildenafil (Viagra) was effective in 79% of male patients with ED after rectal excision for cancer, the effect of these drugs depends on intact pelvic splanchnic nerves [24]. Therefore, nerve preservation during rectal cancer surgery needs to be given greater emphasis in surgical practice. SD can be influenced postoperatively by factors other than surgery alone, such as the recovery period after surgery, coping with disease, and body image. These factors are temporary and do not influence the results after a long-term follow-up. In the case of partial nerve damage, initial dysfunction can be temporarily reduced, but recovery is possible in the first years after the initial operation. However, ED in male rectal-resection patients persists in time [25]. Prospective studies indicate that other indices of quality of life typically improve over time, yet sexual function remains impaired [26, 27].
In conclusion, this study showed that rectal cancer survivors had a high rate of SD, which was seldom treated. Female patients reported higher rates of SD than male patients. These results imply that attention needs to be drawn to SD among male and female survivors of rectal cancer in both research and clinical practice.
References
1. Bloom JR, Petersen DM, Kang SH. Multi-dimensional quality of life among long-term (5+ years) adult cancer survivors. Psychooncology 2007;16:691ŌĆō706. PMID: 17628036.


2. Desnoo L, Faithfull S. A qualitative study of anterior resection syndrome: the experiences of cancer survivors who have undergone resection surgery. Eur J Cancer Care (Engl) 2006;15:244ŌĆō251. PMID: 16882120.


3. Lange MM, van de Velde CJ. Urinary and sexual dysfunction after rectal cancer treatment. Nat Rev Urol 2011;8:51ŌĆō57. PMID: 21135876.


4. Vironen JH, Kairaluoma M, Aalto AM, Kellokumpu IH. Impact of functional results on quality of life after rectal cancer surgery. Dis Colon Rectum 2006;49:568ŌĆō578. PMID: 16583289.


5. Milbury K, Cohen L, Jenkins R, Skibber JM, Schover LR. The association between psychosocial and medical factors with long-term sexual dysfunction after treatment for colorectal cancer. Support Care Cancer 2013;21:793ŌĆō802. PMID: 22948439.


6. Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49:822ŌĆō830. PMID: 9187685.


7. Kaplan SA, Reis RB, Kohn IJ, Ikeguchi EF, Laor E, Te AE, et al. Safety and efficacy of sildenafil in postmenopausal women with sexual dysfunction. Urology 1999;53:481ŌĆō486. PMID: 10096370.


8. Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Pena BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 1999;11:319ŌĆō326. PMID: 10637462.


9. Guvel S, Yaycioglu O, Bagis T, Savas N, Bulgan E, Ozkardes H. Factors associated with sexual function in married women. Turkish J Urol 2003;29:43ŌĆō48.
10. Sprangers MA. Quality-of-life assessment in oncology: achievements and challenges. Acta Oncol 2002;41:229ŌĆō237. PMID: 12195741.


11. Hassan I, Cima RR. Quality of life after rectal resection and multimodality therapy. J Surg Oncol 2007;96:684ŌĆō692. PMID: 18081167.


12. Nesbakken A, Nygaard K, Bull-Njaa T, Carlsen E, Eri LM. Bladder and sexual dysfunction after mesorectal excision for rectal cancer. Br J Surg 2000;87:206ŌĆō210. PMID: 10671929.


13. Havenga K, Enker WE, McDermott K, Cohen AM, Minsky BD, Guillem J. Male and female sexual and urinary function after total mesorectal excision with autonomic nerve preservation for carcinoma of the rectum. J Am Coll Surg 1996;182:495ŌĆō502. PMID: 8646349.

14. Marijnen CA, van de Velde CJ, Putter H, van den Brink M, Maas CP, Martijn H, et al. Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol 2005;23:1847ŌĆō1858. PMID: 15774778.


15. Eveno C, Lamblin A, Mariette C, Pocard M. Sexual and urinary dysfunction after proctectomy for rectal cancer. J Visc Surg 2010;147:e21ŌĆōe30. PMID: 20587375.

16. Hendren SK, O'Connor BI, Liu M, Asano T, Cohen Z, Swallow CJ, et al. Prevalence of male and female sexual dysfunction is high following surgery for rectal cancer. Ann Surg 2005;242:212ŌĆō223. PMID: 16041212.



17. Maurer CA, Z'Graggen K, Renzulli P, Schilling MK, Netzer P, Buchler MW. Total mesorectal excision preserves male genital function compared with conventional rectal cancer surgery. Br J Surg 2001;88:1501ŌĆō1505. PMID: 11683749.


18. Ameda K, Kakizaki H, Koyanagi T, Hirakawa K, Kusumi T, Hosokawa M. The long-term voiding function and sexual function after pelvic nerve-sparing radical surgery for rectal cancer. Int J Urol 2005;12:256ŌĆō263. PMID: 15828952.


19. Havenga K, Maas CP, DeRuiter MC, Welvaart K, Trimbos JB. Avoiding long-term disturbance to bladder and sexual function in pelvic surgery, particularly with rectal cancer. Semin Surg Oncol 2000;18:235ŌĆō243. PMID: 10757889.


20. Lindsey I, Guy RJ, Warren BF, Mortensen NJ. Anatomy of Denonvilliers' fascia and pelvic nerves, impotence, and implications for the colorectal surgeon. Br J Surg 2000;87:1288ŌĆō1299. PMID: 11044153.


21. Hida K, Hasegawa S, Kataoka Y, Nagayama S, Yoshimura K, Nomura A, et al. Male sexual function after laparoscopic total mesorectal excision. Colorectal Dis 2013;15:244ŌĆō251. PMID: 22776077.


22. Masui H, Ike H, Yamaguchi S, Oki S, Shimada H. Male sexual function after autonomic nerve-preserving operation for rectal cancer. Dis Colon Rectum 1996;39:1140ŌĆō1145. PMID: 8831531.


23. Heriot AG, Tekkis PP, Fazio VW, Neary P, Lavery IC. Adjuvant radiotherapy is associated with increased sexual dysfunction in male patients undergoing resection for rectal cancer: a predictive model. Ann Surg 2005;242:502ŌĆō510. PMID: 16192810.



24. Lindsey I, George B, Kettlewell M, Mortensen N. Randomized, double-blind, placebo-controlled trial of sildenafil (Viagra) for erectile dysfunction after rectal excision for cancer and inflammatory bowel disease. Dis Colon Rectum 2002;45:727ŌĆō732. PMID: 12072621.


25. Doeksen A, Gooszen JA, van Duijvendijk P, Tanis PJ, Bakx R, Slors JF, et al. Sexual and urinary functioning after rectal surgery: a prospective comparative study with a median follow-up of 8.5 years. Int J Colorectal Dis 2011;26:1549ŌĆō1557. PMID: 21922200.



26. Camilleri-Brennan J, Steele RJ. Prospective analysis of quality of life and survival following mesorectal excision for rectal cancer. Br J Surg 2001;88:1617ŌĆō1622. PMID: 11736975.


27. Allal AS, Gervaz P, Gertsch P, Bernier J, Roth AD, Morel P, et al. Assessment of quality of life in patients with rectal cancer treated by preoperative radiotherapy: a longitudinal prospective study. Int J Radiat Oncol Biol Phys 2005;61:1129ŌĆō1135. PMID: 15752893.


- TOOLS
-
METRICS

- Related articles in ACP
-
What Should Be Considered for Local Excision in Early Rectal Cancer?2019 August;35(4)
Long-term Outcomes of Laparoscopic Surgery for Colorectal Cancer2011 April;27(2)
Prognostic Role of MMPs in Colorectal Cancer2011 June;27(3)
Sexual Function After a Proctectomy for the Treatment of Rectal Cancer2014 October;30(5)