- Search
| Ann Coloproctol > Volume 36(4); 2020 > Article |
|
See commentary "Surgical Treatment of Upper Gastrointestinal Tract Crohn Disease: A Long Way to Go to Identify the Optimal Method" in Volume 36 on page 207.
Abstract
Purpose
Upper gastrointestinal (GI) tract involvement in Crohn disease (CD) is rare and effectiveness of surgical treatment is limited. The aim of this study was to evaluate characteristics and surgical outcomes of upper GI CD.
Methods
Medical records of 811 patients who underwent intestinal surgery for CD between January 2006 and December 2015 at a single institution were reviewed. Upper GI CD was defined by involvement of the stomach to the fourth portion of duodenum, with or without concomitant small/large bowel CD involvement according to a modification of the Montreal classification.
Results
We identified 24 patients (21 males, 3 females) who underwent surgery for upper GI CD. The mean age at diagnosis was 27 ± 12 years, the mean age at surgery was 33 ± 11 years, and the mean duration of CD was 73.6 ± 56.6 months. Fifteen patients (62.5%) had history of previous perianal surgery. Ten patients (41.7%) had duodenal or gastric stricture and 14 patients (58.3%) had penetrating fistula; patients with fistula were significantly more likely to develop complications (57.1% vs. 20.0%, P = 0.035). One patient with stricture had surgical recurrence. In seven patients with fistula, fistula was related to previous anastomosis. Patients with fistula had significantly longer hospital stays than those with stricture (16 days vs. 11 days, P = 0.01).
Crohn disease (CD) is an inflammatory condition, resulting from stricturing and penetrating complications that can affect any site along the gastrointestinal (GI) tract [1, 2]. The most common CD site is the terminal ileum with or without the ileocecal valve, cecum, or proximal ascending colon [3, 4], while involvement of the upper GI tract, stomach, and duodenum is rare [5-7]. Since the first reported case of upper GI tract involvement by Gottlieb [8] in 1937, upper GI CD has only been found in 0.3%–5% of patients with CD [7, 9-13].
CD is divided into 4 types according to location: ileal (L1), colonic (L2), ileocolonic (L3), and isolated upper disease (L4) [2,14]. From the Montreal revision of the Vienna classification, L4 is defined as upper GI involvement of CD with or without concomitant L1–3 disease [2]. L4 remains poorly understood due to its rarity. While surgery has been suggested as a safe option for patients with intractable duodenal CD [10], surgeries are still performed according to the surgeons’ experience due to the lack of documented studies and guidance.
The most frequent symptoms in patients with upper GI CD are related to obstruction, such as early satiety, nausea, distension, or vomiting [11, 15]. Although most symptoms are associated with obstruction, some patients experience frequent diarrhea or weight loss related to gastro- or duodeno-colic fistula. Depending on the type of CD involvement, different treatment strategies can be pursued.
The purpose of the current study is to evaluate the clinical characteristics and surgical outcomes of upper GI CD and to compare those of upper GI CD and CD at other sites.
We retrospectively evaluated medical records of patients who underwent intestinal surgery for CD at Asan Medical Center, Seoul, Korea between January 2006 and December 2015. Patients were included if they had undergone bowel resection, strictureplasty, or bypass surgery for CD during the study period and were 16 years of age or older at the time of surgery. Patients were excluded if surgery was only open biopsy, stoma formation, or restoration of stoma; if there was a concurrent or prior history of other malignancies; or if CD could not clarify the location due to ambiguous description or absence of location information on medical record. The following variables from the medical records of the study cohort were collected: demographic characteristics (sex, age at diagnosis and at surgery, duration and follow-up of CD, family history, history of smoking, previous perianal and abdominal surgery); preoperative characteristics (indication for surgery; Montreal classification; and preoperative medications including steroids, immunomodulators, and anti-tumor necrosis factor alpha); operative details (operative approach; timing of surgery including whether the procedure was elective or emergency; methods of anastomosis; and anastomosis shapes including side-to-side, endto-side, or end-to-end); and postoperative outcomes (duration of postoperative hospital stay, clinical recurrence and surgical recurrence of CD, and postoperative complications by postoperative day 30).
The study protocol was approved by the Institutional Review Board of the Asan Medical Center (IRB No. 2019-0428), and the study was performed in accordance with the Declaration of Helsinki.
Upper GI CD was defined as L4 according to the Montreal classification and gastric or duodenal fistula related to anastomosis at other sites. For subgroup analysis, patients with upper GI CD involvement were classified as either stricturing group or penetrating group according to the indication of surgery. Other site CD was defined as any kind of CD involvement apart from upper GI CD.
CD location was assessed based on pathological, intraoperative, and imaging findings using a small bowel series (SBS), computed tomography enterography (CTE), magnetic resonance imaging (MRI), and colonoscopy. Medical treatment of CD was based on a step-up approach, and more potent therapies were added if and when patients became unresponsive to first-line or less toxic agents, as mentioned in a previous report from our institution [4]. During medical treatment, the gastroenterologist consulted with specialized colorectal surgeons when considering surgery.
Postoperative complications were defined as grade 2A or higher on the Clavien-Dindo classification after surgery [16]. Postoperative complications were classified as noninfectious or infectious. Non-infectious complications included postoperative ileus; bleeding at the anastomosis site or an intraabdominal hematoma that required transfusion, pig-tail drainage or similar intervention, surgery, or close monitoring due to massive hematochezia (> 3 times per a day); a thromboembolic event such as deep vein thrombosis or pulmonary thromboembolism; pleural effusion; and organ dysfunction. Infectious complications included intraabdominal sepsis (anastomotic leakage and intraabdominal abscess determined by an obvious change in drainage or abdominopelvic computed tomography), entero-cutaneous fistula, wound infection, and extra-abdominal infections (sepsis and urinary and respiratory tract infections).
Recurrences were divided into clinical and surgical recurrence. Clinical recurrence was defined as a definite change in imaging studies (SBS, CTE, or MRI) or colonoscopy following the onset of symptoms in a patient (e.g., any type of fistula with an abscess on imaging or a stricture or an ulcer that was difficult to pass by colonoscopy). Surgical recurrence was defined as any repeated surgery on any section of the bowel for pathologically confirmed CD. Repeated operations for pathologically confirmed anastomotic disease, including cases in which the small bowel was diseased at the anastomosis or the stoma site, were also defined as surgical recurrences.
Discrete variables including demographic and preoperative characteristics, operative methods, and operative details were analyzed using the chi-squared test comparing upper GI CD with other sites. Continuous variables including age at surgery, hospital stay duration, and duration of follow-up after surgery were compared using unpaired Student t-test or the Mann-Whitney U-test as appropriate. Statistical significance was defined as P< 0.05, and all statistical analyses were performed using IBM SPSS Statistics ver. 21.0 (IBM Corp., Armonk, NY, USA).
Of 811 patients, 339 patients (41.8%) had ileal involvement, 54 patients (6.7%) had colonic involvement, and 387 patients (47.7%) had ileo-colonic involvement. Only 24 patients (2.96%) met the criteria for upper GI CD. The ratio of males to females in the study cohort was 7:1. The mean ages at diagnosis and at operation in the upper GI CD patients were 27± 11.8 years and 32.8± 10.8 years, respectively. Patients with upper GI CD had more smoking history (45.8% ex-smoker and 12.5% current smoker) and perianal surgery (62.5%) than patients with CD at other sites (24.3% ex-smoker, 9.5% current smoker, P = 0.035 and 40.6% perianal surgery, P= 0.036). Among the 24 patients with upper GI CD, the proportion of concomitant other site involvement was lower in L1 and higher in L2 and L3 (16.7% L1, 16.7% L2, and 66.7% L3, P= 0.015) compared with the proportion of CD at other sites. Variables that did not differ significantly between these 2 groups included age at diagnosis and operation, sex, duration of disease, follow-up duration, body mass index at the time of operation, family history of CD, age and disease behavior by Montreal classification, and prescribed medication (Table 1).
All but one patient with upper GI CD underwent open surgery, while 21.3% of patients with CD at other sites underwent laparoscopic surgery (P= 0.008). All patients with upper GI CD underwent hand-sewn anastomosis, and more patients with upper GI CD underwent hand-sewn anastomosis for concomitant other site CD than patients with CD at other sites (16.7% vs. 3.0%, P = 0.006). Four patients with upper GI CD received ileostomy for ileocolic involvement of CD. The rate of emergency operation and anastomosis types were not significantly different between patients with upper GI CD and patients with CD at other sites (Table 2).
Patients with upper GI CD required more packed red blood cell transfusion (7.04± 18.24 units vs. 1.64± 3.84 units, P= 0.001), longer operation time (196± 68 minutes vs. 158± 64 minutes, P= 0.013), and longer hospital stays than patients with CD at other sites (27.5± 49.8 days vs. 12.8± 13.0 days, P= 0.001). In terms of complications, patients with upper GI CD experienced more anastomotic leakage (16.7% vs. 3.6%, P= 0.012) and more postoperative ileus (12.5% vs. 2.8%, P= 0.034). More patients with upper GI CD experienced clinical and surgical recurrence compared with patients with CD at other sites (clinical recurrence: 62.5% vs. 59.3%, P= 0.001; surgical recurrence: 16.7% vs. 11.4%, P= 0.001). There was no significant difference in mean time to clinical and surgical difference between the upper GI CD group and the other site CD group (Tables 3, 4).
In the subgroup analysis, stricturing was indicated in 10 patients (45%) and penetrating was diagnosed 14 patients (55%). Stricturing was identified in gastric (6 patients, 66.7%) and duodenal (4 patients, 33.3%) locations. The penetrating group included fistula related to previous anastomosis (7 patients, 54.5%), gastric fistula (2 patients, 45.5%), and duodenal fistula (5 patients, 45.5%) (Fig. 1). Though not statistically significant, a higher proportion of patients in the penetrating group developed infectious complications such as anastomotic leak than patients in the stricturing group (42.9% vs. 10%, P= 0.07). One patient (10.0%) in the stricturing group had a surgical complication requiring a second anastomosis procedure. Two patients (20.8%) with duodenal fistula in the penetrating group needed an additional primary repair procedure with or without feeding jejunostomy (Fig. 1).
The current study investigated clinical characteristics, surgical management, and operative outcomes of patients with upper GI CD. Previous retrospective studies showed a prevalence of 0.3%– 5% [7, 9-13], while the few prospective studies to analyze CD presentation after esophagogastroduodenoscopy in upper GI CD have suggested a prevalence of 24% to 56% [17, 18]. We found the prevalence of upper GI CD to be 2.96%, in line with other retrospective studies that reported using surgical data [10-13] and endoscopic data [19]. Although the previous prospective studies found quite high prevalence (~56%) from endoscopy and endoscopic biopsy [17, 18], the current study showed that the rate of patients who needed surgery was just around 3%. The majority of patients in our study experienced frequent stool passage or diarrhea related to penetrating fistula, especially those related to previous anastomosis (29.2%), followed by symptoms reported in other studies [17, 20] such as nausea, vomiting, and indigestion.
We found that patients with upper GI CD were significantly more likely to be male and to have a history of perianal surgery and smoking than patients with CD at other sites. Although male dominance of CD has been previously reported in Korea—unlike the relatively similar sex ratio in Western countries [21-23]—most upper GI CD patients were male but differences in sex were not statistically significant in the multivariate analysis (P = 0.084). One study reported that CD with perianal disease was associated with a more severe course of disease [24]. In Korea, perianal CD involvement had high prevalence at around 40%, compared with a prevalence of around 20% in Western countries [4, 25]. It is well known that smoking is associated with CD [26, 27]. Considering our findings, smoking cessation and education related to smoking may help prevent the development of upper GI CD.
Options for surgical management of complicated upper GI CD include bypass, resection, or strictureplasty [10]. Unlike the management of CD in other parts of the GI tract, bypass is favored over resection for upper GI CD [10]. This may be due to the anatomical differences and the complexity of upper GI resection. The results of the current study were similar and all patients with stricturing CD received bypass surgery (78.6% patients with penetrating CD primary repair and 21.4% patients with penetrating CD bypass surgery). Considering the relatively good postoperative results of the current study, surgical procedures should be selected after consideration of the characteristics, location, and behavior of CD. Some studies have found that resection has four times the morbidity of bypass surgery and have insisted that bypass with or without vagotomy and strictureplasty is preferable for the treatment of intractable duodenal CD [5, 10]. Previous studies have attempted to examine the appropriateness of surgical treatment of duodenal CD, but they have not yet identified the treatment of choice [5]. Although laparoscopic surgery is recently increasing even in CD [28], the current study showed that only one patient with upper GI CD (4.2%) received laparoscopic surgery due to high rate of reoperation (45.8%) and complexity of surgery (100% concomitant other site CD).
The current study found that patients with upper GI CD required more transfusion, had longer operation time and hospital stays, experienced more anastomotic leak and postoperative ileus, and had higher rates of clinical and surgical recurrences than patients with CD at other sites, which is in line with other studies [5,29]. The current study also found that more patients in the penetrating group experienced postoperative complications than did patients in the stricturing group. However, one study revealed that patients with upper GI CD seemed to show an even better outcome regarding development of intestinal fistula (penetrating disease) [5]. Although that study found good outcomes in patients with penetrating fistula, considering the complexity of surgery related to anatomical difficulty of duodenal surgery and needs of more anastomosis in aspect of the anastomosis of the affected bowel sites, appropriate surgery would be needed. Besides, the current study included patients with previous anastomosis site fistula, and these patients did not need reoperation, even primary closure (Fig. 1). Although there was no institutional strategy for primary repair of upper GI fistula, this result implies that fistula related to a previous anastomosis site produced good outcome even in primary closure.
Because this study was retrospective in design, we could not completely control for patient characteristics. However, this study not only assessed upper GI CD, but also other site CD characteristics, surgical methods, and postoperative outcomes. Another limitation of this study was the lack of analysis of endoscopic findings. Nevertheless, the present study is meaningful in identifying the incidence of upper GI CD among patients who underwent intestinal surgery with a relatively larger number than other retrospective studies.
In conclusions, CD patients with upper GI involvement had low prevalence, a relatively low rate of surgical recurrence, and prolonged hospital stays after surgery. The penetrating group had more complications than the stricturing group.
Fig. 1.
Classification and operative details of the upper gastrointestinal Crohn disease patients. GI, gastrointestinal; Pt, patient; GJ, gastrojejunostomy; JJstomy, jejunojejunostomy; 1’ repair, primary repair; DJ, duodenojejunostomy; SB R&A, small bowel resection and anastomosis.
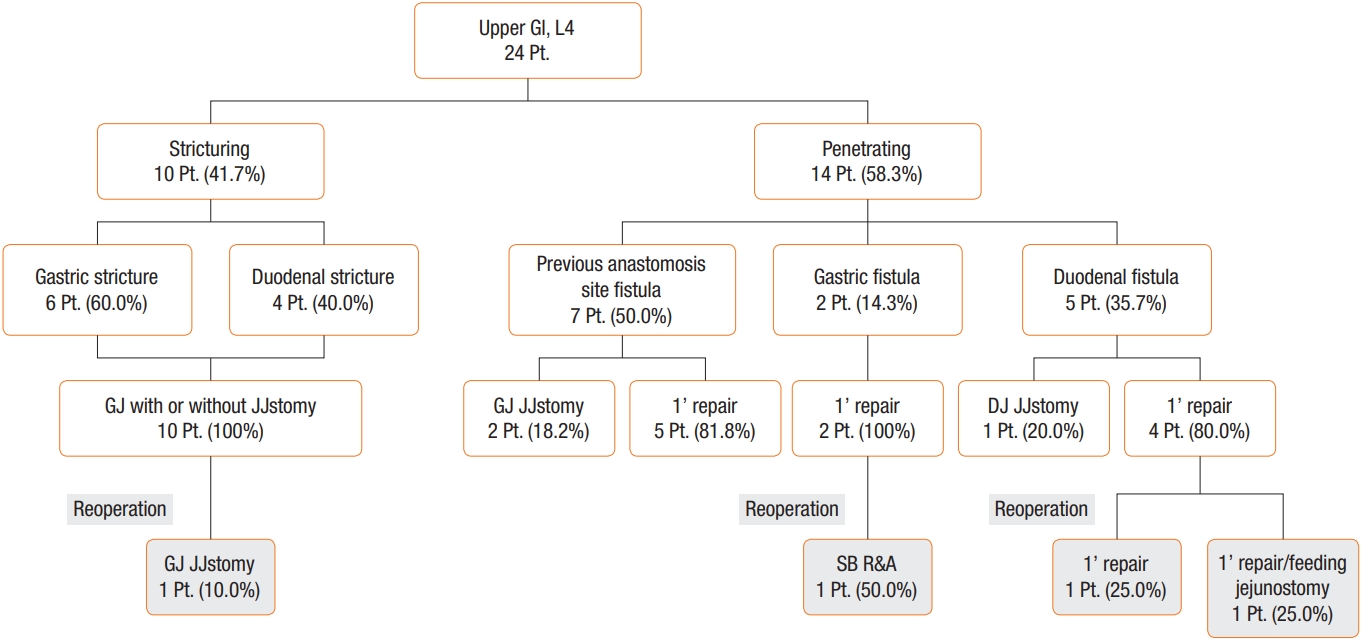
Table 1.
Demographic characteristics of patients with CD in the upper GI tract and other sites
| Variable | Upper GI CD (n = 24) | Other site CD (n = 787) | P-value |
|---|---|---|---|
| Age at diagnosis (yr) | 27 ± 12 | 26 ± 10 | 0.75 |
| Age at operation (yr) | 33 ± 11 | 32 ± 10 | 0.61 |
| Sex, female : male | 3 (12.5) : 21 (87.5) | 233 (29.6) : 554 (70.4) | 0.07 |
| Duration of disease (mo) | 73.6 ± 56.6 | 69.7 ± 61.1 | 0.87 |
| Follow-up duration (mo) | 147.1 ± 59.7 | 145.1 ± 69.5 | 0.88 |
| Body mass index at operation (kg/m2) | 17.76 ± 2.27 | 18.55 ± 3.10 | 0.11 |
| Hospital stay (day) | 36.6 ± 58.4 | 19.4 ± 16.6 | 0.001* |
| Family history of CD, yes | 0 (0) | 24 (3.0) | 0.48 |
| History of smoking | 0.035* | ||
| None | 10 (41.7) | 521 (66.2) | |
| Ex-smoker | 11 (45.8) | 191 (24.3) | |
| Current smoker | 3 (12.5) | 75 (9.5) | |
| Previous abdominal surgery, yes | 11 (45.8) | 266 (33.7) | 0.45 |
| Previous perianal surgery, yes | 15 (62.5) | 319 (40.6) | 0.036* |
| History of fistula-in-ano | 0.06 | ||
| Yes | 17 (70.8) | 393 (49.9) | |
| No | 7 (29.2) | 394 (50.1) | |
| Montreal classification behavior | 0.76 | ||
| Nonstricturing, nonpenetrating (B1) | 2 (8.3) | 42 (5.4) | |
| Stricturing (B2) | 10 (41.7) | 270 (34.3) | |
| Penetrating (B3) | 12 (50.0) | 475 (60.3) | |
| Montreal classification location | 0.015* | ||
| Ileocolic (L1) | 4 (16.7)a | 346 (43.9) | |
| Colic (L2) | 4 (16.7)a | 54 (6.9) | |
| Ileal (L3) | 16 (66.7)a | 387 (49.2) | |
| Medication | |||
| 5-Amino-salicylic acid or no medication | 8 (33.3) | 339 (43.1) | 0.21 |
| IMM | 10 (41.7) | 243 (30.9) | 0.27 |
| Anti-TNF-a | 1 (4.2) | 48 (6.1) | 0.57 |
| Steroid | 0 (0) | 42 (5.3) | 0.63 |
| IMM+TNF | 3 (12.5) | 54 (6.9) | 0.24 |
| IMM+steroid | 2 (8.3) | 41 (5.2) | 0.37 |
| Steroid+TNF | 0 (0) | 8 (1.0) | 0.79 |
| All combination | 0 (0) | 12 (1.5) | 0.70 |
Table 2.
Operative details of patients with CD in the upper GI tract and other sites
| Variable | Upper GI CD (n = 24) | Other site CD (n = 787) | P-value |
|---|---|---|---|
| Emergency operation, yes | 1 (4.2) | 70 (8.9) | 0.710 |
| Operative approach | 0.008* | ||
| Open | 23 (95.8) | 619 (78.7) | |
| Laparoscopy | 1 (4.2) | 168 (21.3) | |
| Anastomosis method | 0.006* | ||
| Hand-sewn | 24 (100)/4 (16.7)a | 23 (3.0) | |
| Stapled | 0 (0)/16 (66.7)a | 639 (82.0) | |
| Stoma formation | 0 (0)/4 (16.7)a | 117 (15.0) | |
| Anastomosis type | 0.580 | ||
| Side-to-side, functional end-to-end | 13 (54.2)/17 (70.8)a | 565 (72.5) | |
| End-to-side | 0 (0)/1 (4.2)a | 61 (7.8) | |
| End-to-end | 11 (45.8)/2 (8.3)a | 36 (4.6) | |
| Stoma formation | 0 (0)/4 (16.7)a | 117 (15.0) |
Table 3.
Operative outcomes of patients with CD in the upper GI tract and other sites
| Variable | Upper GI CD (n = 24) | Other site CD (n = 787) | P-value |
|---|---|---|---|
| Postoperative pRBC transfusion (unit) | 7.04 ± 18.24 | 1.64 ± 3.84 | 0.001* |
| Operative time (min) | 196 ± 68 | 158 ± 64 | 0.013* |
| Hospital stay after surgery (day) | 27.5 ± 49.8 | 12.8 ± 13.0 | 0.001* |
| Total complications | 10 (41.7) | 193 (24.6) | 0.16 |
| Intra-abdominal infection | 5 (20.8) | 72 (9.2) | 0.07 |
| Bleeding | 0 (0) | 23 (2.9) | 0.72 |
| Wound | 2 (8.3) | 70 (8.9) | 0.64 |
| Anastomotic leak | 4 (16.7) | 28 (3.6) | 0.012* |
| Postoperative ileus | 3 (12.5) | 22 (2.8) | 0.034* |
| Entero-cutaneous fistula | 0 (0) | 4 (0.5) | 0.89 |
| Clinical recurrence, yes | 15 (62.5) | 467 (59.3) | 0.001* |
| Mean time to clinical recurrence (mo) | 39 ± 23 | 35 ± 27 | 0.54 |
| Surgical recurrence, yes | 4 (16.7) | 90 (11.4) | 0.001* |
| Mean time to surgical recurrence (mo) | 77 ± 19 | 49 ± 34 | 0.055 |
Table 4.
Postoperative hospital stay and complications of patients with CD in the upper GI tract and other sites
| Variable | Stricture (n = 10) | Penetrating (n = 14) | P-value |
|---|---|---|---|
| Hospital stay after surgery (day) | 11 (8−15) | 16 (12−30) | 0.01* |
| Overall complication | 2 (20.0) | 8 (57.1) | 0.035* |
| Intraabdominal abscess | 0 | 4 (28.6) | 0.55 |
| Wound | 0 | 2 (27.3) | 0.22 |
| Anastomotic leak | 2 (22.2) | 1 (9.1) | 0.58 |
| Postoperative ileus | 1 (11.1) | 1 (9.1) | 0.71 |
REFERENCES
1. Lock MR, Farmer RG, Fazio VW, Jagelman DG, Lavery IC, Weakley FL. Recurrence and reoperation for Crohn’s disease: the role of disease location in prognosis. N Engl J Med 1981;304:1586–8.


2. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006;55:749–53.


3. Farmer RG, Whelan G, Fazio VW. Long-term follow-up of patients with Crohn’s disease: relationship between the clinical pattern and prognosis. Gastroenterology 1985;88:1818–25.


4. Park SH, Yang SK, Park SK, Kim JW, Yang DH, Jung KW, et al. Long-term prognosis of Crohn’s disease and its temporal change between 1981 and 2012: a hospital-based cohort study from Korea. Inflamm Bowel Dis 2014;20:488–94.


5. Lazarev M, Huang C, Bitton A, Cho JH, Duerr RH, McGovern DP, et al. Relationship between proximal Crohn’s disease location and disease behavior and surgery: a cross-sectional study of the IBD Genetics Consortium. Am J Gastroenterol 2013;108:106–12.



6. Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol 2010;105:289–97.



7. Fielding JF, Toye DK, Beton DC, Cooke WT. Crohn’s disease of the stomach and duodenum. Gut 1970;11:1001–6.



8. Gottlieb CH. Regional jejunitis. Am J Roetgenol 1937;38:881–3.
9. Wagtmans MJ, Verspaget HW, Lamers CB, van Hogezand RA. Clinical aspects of Crohn’s disease of the upper gastrointestinal tract: a comparison with distal Crohn’s disease. Am J Gastroenterol 1997;92:1467–71.

10. Shapiro M, Greenstein AJ, Byrn J, Corona J, Greenstein AJ, Salky B, et al. Surgical management and outcomes of patients with duodenal Crohn’s disease. J Am Coll Surg 2008;207:36–42.


11. Nugent FW, Roy MA. Duodenal Crohn’s disease: an analysis of 89 cases. Am J Gastroenterol 1989;84:249–54.

12. Gong J, Wei Y, Gu L, Li Y, Guo Z, Sun J, et al. Outcome of surgery for coloduodenal fistula in Crohn’s disease. J Gastrointest Surg 2016;20:976–84.



13. Wilk PJ, Fazio V, Turnbull RB Jr. The dilemma of Crohn’s disease: ileoduodenal fistula complicating Crohn’s disease. Dis Colon Rectum 1977;20:387–92.


14. Gasche C, Scholmerich J, Brynskov J, D’Haens G, Hanauer SB, Irvine EJ, et al. A simple classification of Crohn’s disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis 2000;6:8–15.


15. Lightner AL, Fletcher JG. Duodenal Crohn’s disease: a diagnostic conundrum. J Gastrointest Surg 2018;22:761–3.



16. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13.



17. Korelitz BI, Waye JD, Kreuning J, Sommers SC, Fein HD, Beeber J, et al. Crohn’s disease in endoscopic biopsies of the gastric antrum and duodenum. Am J Gastroenterol 1981;76:103–9.

18. Alcantara M, Rodriguez R, Potenciano JL, Carrobles JL, Munoz C, Gomez R. Endoscopic and bioptic findings in the upper gastrointestinal tract in patients with Crohn’s disease. Endoscopy 1993;25:282–6.



19. Rutgeerts P, Onette E, Vantrappen G, Geboes K, Broeckaert L, Talloen L. Crohn’s disease of the stomach and duodenum: a clinical study with emphasis on the value of endoscopy and endoscopic biopsies. Endoscopy 1980;12:288–94.



20. Diaz L, Hernandez-Oquet RE, Deshpande AR, Moshiree B. Upper gastrointestinal involvement in Crohn disease: histopathologic and endoscopic findings. South Med J 2015;108:695–700.


21. Park SH, Kim YJ, Rhee KH, Kim YH, Hong SN, Kim KH, et al. A 30-year trend analysis in the epidemiology of inflammatory bowel disease in the Songpa-Kangdong District of Seoul, Korea in 1986-2015. J Crohns Colitis 2019;13:1410–7.



22. Limketkai BN, Shah SC, Hirano I, Bellaguarda E, Colombel JF. Epidemiology and implications of concurrent diagnosis of eosinophilic oesophagitis and IBD based on a prospective populationbased analysis. Gut 2019;68:2152–60.


23. Stjarngrim J, Ekbom A, Hammar U, Hultcrantz R, Forsberg AM. Rates and characteristics of postcolonoscopy colorectal cancer in the Swedish IBD population: what are the differences from a nonIBD population? Gut 2019;68:1588–96.


24. Bernell O, Lapidus A, Hellers G. Recurrence after colectomy in Crohn’s colitis. Dis Colon Rectum 2001;44:647–54.


25. Schwartz DA, Tagarro I, Carmen Diez M, Sandborn WJ. Prevalence of fistulizing Crohn’s disease in the United States: estimate from a systematic literature review attempt and population-based database analysis. Inflamm Bowel Dis 2019;25:1773–9.




26. Cottone M, Rosselli M, Orlando A, Oliva L, Puleo A, Cappello M, et al. Smoking habits and recurrence in Crohn’s disease. Gastroenterology 1994;106:643–8.


27. Cosnes J, Beaugerie L, Carbonnel F, Gendre JP. Smoking cessation and the course of Crohn’s disease: an intervention study. Gastroenterology 2001;120:1093–9.










